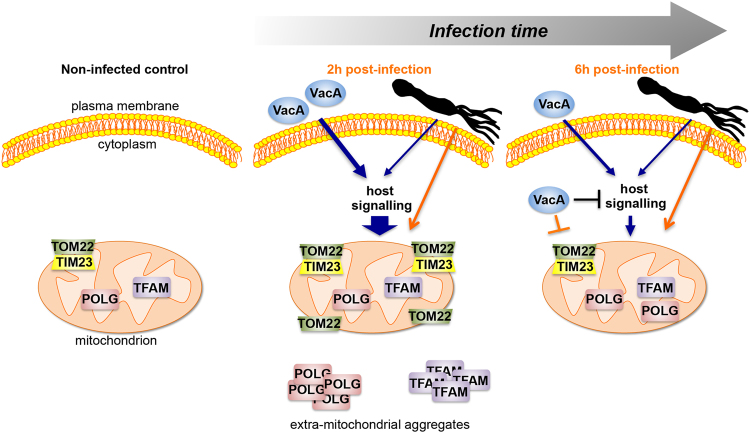
Figure 7.
Schematic summary of VacA-mediated and VacA-independent mitochondrial alterations upon H. pylori infection. Left panel: non-infected cells with regular levels and localization of translocases subunits TOM22 and TIM23, DNA polymerase POLG, and transcription and DNA maintenance factor TFAM. Middle panel: during the first 2 hours of infection, extracellular VacA interacts with the epithelial cell surface activating host signaling pathways (we cannot exclude that undetectable levels of the VacA are present intracellularly and may also affect the levels of mitochondrial factors). This signaling results in enriched (large blue arrow) mitochondrial translocase subunits, in particular TOM22, dramatically induced POLG and TFAM, and doubled mtDNA content (not shown). At this stage, POLG and TFAM are largely extramitochondrial. These events are transitory, and are reduced or return to control levels (6 h pi, right panel) in the presence of intracellular VacA, which may inhibit (a blocked black line) and/or counteract (a blocked orange line) the signal(s) leading to mitochondrial alterations. TOM22, TIM23, POLG, and TFAM increase also in the absence of VacA, although at a lower extent, suggesting the involvement of unknown H. pylori activities (indicated with a schematic bacterium), not yet identified. These H. pylori activities may act through the same host signaling as VacA and/or through other interactions (orange arrow). VacA-independent alterations induce mitochondrial biogenesis with no increase of mtDNA content (not shown), and POLG and TFAM levels that remain higher than controls, suggesting a delayed effect than in the presence of VacA. At 48 h (not shown) and independently of the presence of VacA, TOM22, TFAM, POLG return to control levels, eventhough mitochondrial mass and mitochondria fragmentation increase and mtDNA is depleted.