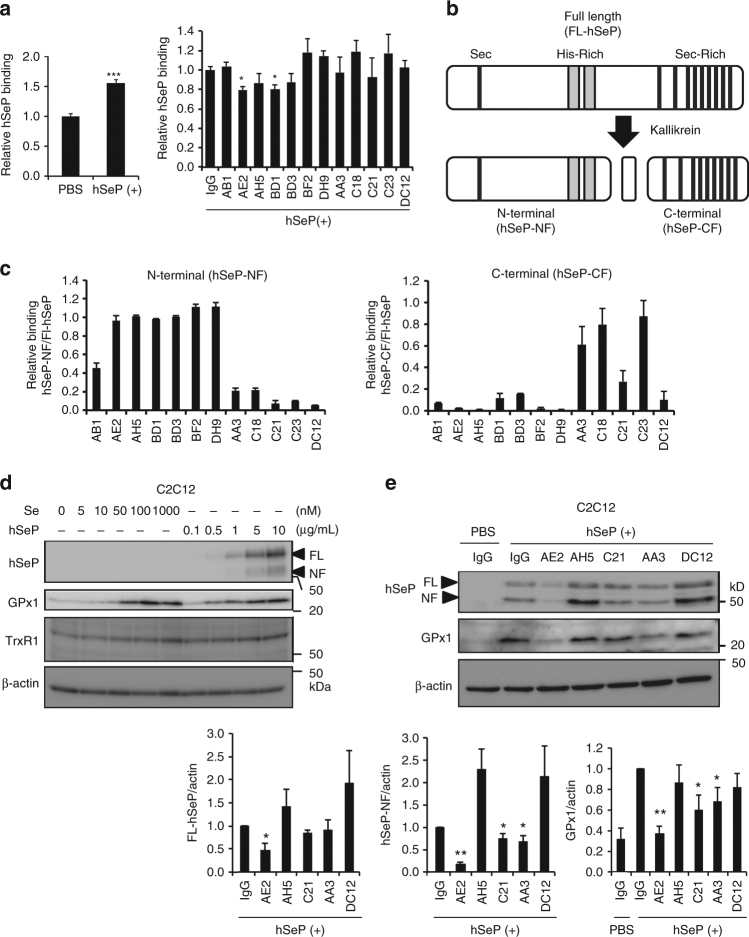
Fig. 1.
Identification of antibodies inhibiting the binding and selenium supply of SeP. a Inhibitory effects of monoclonal antibodies on the interaction between undifferentiated C2C12 cells and human SeP. C2C12 cells were incubated with purified human SeP (hSeP) protein (0.5 µg/mL) in the absence (left panel) or the presence (right panel) of each mAb (10 µg/mL) at 4 °C, and then SeP binding was analysed (n = 3, means ± s.d.). ***P < 0.001, Student's t-test (left panel), *P < 0.05, vs. control IgG, Tukey-ANOVA (right panel). b Schematic representation of limited proteolysis of human SeP by plasma kallikrein. The sequential limited proteolysis of full-length-hSeP (FL-hSeP) by plasma kallikrein (R235-Q236 and R242-D243) resulted in the generation of N-terminal (hSeP-NF) and C-terminal fragments (hSeP-CF). c Immunoreactivity of each mAb against hSeP-NF and hSeP-CF was determined by direct ELISA (n = 3, means ± s.d.). Relative binding, hSeP-NF immunoreactivity/FL-hSeP immunoreactivity and hSeP-CF immunoreactivity/FL-hSeP immunoreactivity, is shown. d Differentiated C2C12 myocytes were treated with each concentration of sodium selenite (Se) and hSeP for 24 h, as described in the Methods, and then whole-cell lysates were analysed by western blotting with anti-hSeP Ab BD1, anti-GPx1 Ab and anti-TrxR1 Ab KB12. e Effects of monoclonal antibodies for Se-supply activity of human SeP in C2C12 myocytes. C2C12 myocytes were treated with hSeP (0.5 µg/mL) in the presence of each mAb (10 µg/mL) for 24 h. Human SeP and GPx1 levels in whole-cell lysates were determined by western blotting (n = 3, means ± s.d.). The band intensity of hSeP was only evaluated in hSeP-treated cells. **P < 0.01, *P < 0.05, vs. control IgG of hSeP-treated cells, Tukey-ANOVA