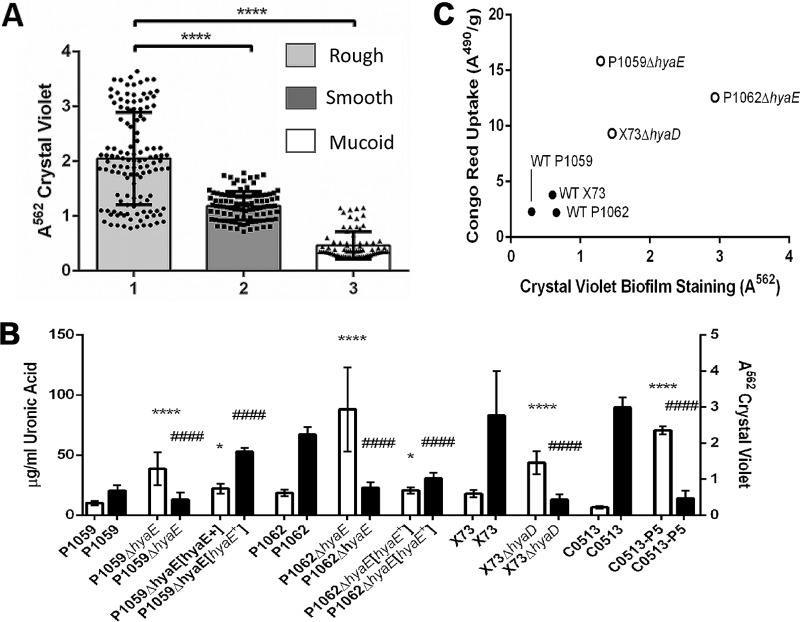
FIG 1 .
Correlation between CPS production and biofilm formation by P. multocida clinical isolates and laboratory strains. (A) Clinical and laboratory isolates were grouped on the basis of their colonial morphology (amount or lack of mucoid appearance and iridescence) on blood agar in relation to biofilm formation. The y axis represents the A562 after staining of the biofilm with CV and solubilization of the residue with 95% ethanol. Groups: 1, rough colonies/unencapsulated; 2, smooth colonies/intermediate encapsulation; 3, mucoid colonies/highly encapsulated. The amount of biofilm formed (as indicated by CV assay) by isolates in groups 2 and 3 was significantly smaller than the amount of biofilm formed by group 1 isolates (****, P ≤ 0.0001). (B) Comparison of biofilm formation by WT strains and the respective isogenic capsule-deficient mutants or an in vitro-passaged variant of WT C0153 (C0153-P5). The amounts of biofilm and CPS were determined by CV assay and uronic acid assay, respectively. The WT strains and the respective capsule-deficient mutants are listed on the x axis. The left y axis represents the concentration (μg/ml) of the uronic acid removed from the cell surface. The right y axis is the absorbance of solubilized CV after staining. White bars indicate the absorbance value from CV staining; black bars indicate uronic acid content. Biofilm formation was significantly higher in isolates producing less CPS. Significant differences between the parent and mutant strains in the CV assay are indicated by asterisks, and those in the uronic acid assay are indicated by number signs as follows: *, P ≤ 0.05; **** or ####, P ≤ 0.0001. (C) Correlation plot of P. multocida CR uptake absorbance values (y axis) and CV absorbance values for biofilms (x axis). The Pearson correlation coefficient is 0.7324 for all values and 0.9635 if P1059ΔhyaE is excluded. Encapsulated isolates are represented by solid dots, while acapsular isolates are represented by hollow dots.