Abstract
Telomere shortening is associated with colorectal carcinogenesis and recent studies have focused on its characteristics in both normal mucosa and tumor tissues. To clarify the role of telomeres in colorectal carcinogenesis, we analyzed telomere shortening in normal and tumor regions of 93 colorectal precursor lesions. Telomere length was examined in 61 tubular adenomas (TAs) and 32 serrated polyps (SPs), and PIK3CA expression, KRAS mutation, BRAF mutation, and MSI were also analyzed. Telomere length was similar in normal and tumor tissues of TAs and SPs. In normal tissues of TAs, telomere shortening was associated with PIK3CA amplification (81.3% vs. 18.8%, p < 0.001), whereas it was associated with BRAF mutation in normal tissues of SPs (66.7% vs. 23.1%, p = 0.060). According to the analysis on tumor tissues, KRAS and BRAF mutations were mutually exclusive in TAs and SPs (p < 0.001), and telomere shortening was associated with mitochondrial microsatellite instability (63.6% vs. 36.4%, p = 0.030). These data suggested a pivotal role of telomere shortening in normal colorectal tissue for proceeding to TAs or SPs along with PIK3CA amplification and BRAF mutation, respectively. Moreover, telomeres in TAs may collaborate with mitochondrial instability for disease progression.
Keywords: Telomere shortening, colorectal cancer, tubular adenomas, serrated polyps
Introduction
Most colorectal cancers (CRC) are thought to arise from the adenoma-carcinoma series by sequential accumulation of genetic alterations [1]. Tubular adenomas (TA) progress through APC, KRAS, and p53 mutations whereas serrated polyps (SP) may be associated with microsatellite instability (MSI or nMSI) and BRAF mutations [2-4]. Though the molecular mechanisms of this progression are uncertain, precancerous CRC lesions may have various genetic backgrounds and carcinogenesis processes.
Telomeres, composed of 6-bp TTAGGG repeat sequences, are nucleoprotein complexes capping each end of the eukaryotic chromosome [5]. In normal human somatic cells, telomeres have an average length of 5 to 15 kilobases and are shortened by ~30 to 200 base pairs at every cell division. With continuous shortening, telomeres eventually reach a critical length that triggers replicative senescence and apoptosis [6]. The disruption of this process or telomere dysfunction is considered a major factor for cancer progression [6]. In CRC tissues, telomere length has been found to be short as compared to that in adjacent normal mucosa [7-9]. This telomere shortening may be an early event in colorectal carcinogenesis and was frequently found in adenomas. Recently, the characteristics of telomeres in both normal mucosa and tumor tissues were found to be independently associated with CRC progression [10]. To understand the role of telomeres in colorectal carcinogenesis, telomeres and their associated genes should be studied in tumors and in matched normal mucosa samples of precancerous lesions.
In the present study, we analyzed telomere length in normal and tumor regions of 93 colorectal precursor lesions, comprising 61 TAs and 32 SPs. Based on previous our studies [11,12], to better understanding colorectal carcinogenesis, key molecular markers in CRCs, including nuclear and mitochondrial MSIs, KRAS and BRAF mutations, and PIK3CA amplification, were also analyzed in these lesions.
Materials and methods
Patients and DNA extraction
To obtain precancerous lesions, the records of colonoscopic polypectomies performed at Dongsan Medical Center in the period between 1999 and 2003 were reviewed, and 61 TAs and 32 SPs were selected. The SPs were categorized as serrated adenomas or hyperplastic polyps. Among SPs, mixed form and traditional serrated adenomas were excluded. Exclusion criteria were previous history of surgical resection for CRCs, and evidence of hereditary non-polyposis colorectal cancer (Amsterdam criteria) or familial adenomatous polyposis. Tumor area and adjacent normal mucosa were selected from the slides based on hematoxylin and eosin stained sections. Subsequently, the selected areas from paraffin-embedded tissues were used for DNA extraction using an extraction kit (AbsoluteTM DNA extraction Kit, Bio-Sewoom, Korea) according to the manufacturer’s instructions. Tissue-based specimen collection and studies were approved by the institutional review boards of Keimyung University Dongsan Medical Center, Korea. All participants provided written consent and indicated willingness to donate their tissue samples for research.
Telomere length and PIK3CA expression
Telomere length and PIK3CA expression were analyzed by quantitative real-time PCR. For quantitative determination of telomere length relative to nDNA, primers for specific amplification of telomere (T) and nDNA-encoded ß-globin (S) were selected as reported in a previous study [13]. PIK3CA (T) and ß-globin (S) expression levels were analyzed and relative PIK3CA expression was then calculated.
Real-time PCR was performed on a LightCycler 480 II system (Roche Diagnostics, Germany). Relative telomere length and PIK3CA expression were determined by calculating T/S values using the formula T/S = 2-ΔCt, where ΔCt = average Cttelomere or PIK3CA - average Ctß-globin. Each measurement was repeated in triplicate and 5 serially diluted control samples were included in each experiment. Amplification of PIK3CA was defined by a copy number ≥ 3.
KRAS and BRAF mutations
KRAS mutations in codons 12 and 13, and the BRAF V600E mutation were analyzed by pyrosequencing (PyroMark Q24, Sweden). The primers for amplification and pyrosequencing were designed as described previously [11,12]. The pyrosequencing reaction was performed on a PyroMark Q24 instrument using Pyro Gold Q24 reagents (Qiagen, Netherlands). The pyrosequencing primers were used at a final concentration of 0.3 µmol/L. The resulting data were analyzed and quantified using PyroMark Q24 software version 2.0.6 (Qiagen, Netherlands).
Microsatellite instability (MSI)
Although the National Cancer Institute recommends the Bethesda panel for MSI analysis, recent studies have shown that BAT25 and BAT26 analysis can accurately detect MSI without additional markers [11,12]. Most of the mitochondrial MSI (mtMSI) was found in the D-loop region, especially in the D310 region. Therefore, the MSI was analyzed with BAT25 and BAT26 for nMSI and D310 for mtMSI. PCR amplification was performed using a previously described method [11].
Statistical analysis
The SPSS statistical package, version 19.0 for Windows, was used for all statistical analyses. Chi-square, Fischer’s exact tests, and Spearman correlation analysis were used to analyze the relationship between variables. A two-tailed probability < 0.05 was required for statistical significance.
Results
Telomere length in colorectal precancerous lesions
Telomere length was successfully analyzed using real time PCR in 93 colorectal precursor lesions, comprising of 61 TAs and 32 SPs. Telomere length was calculated in paired normal and tumor tissues. In normal tissues, the average telomere lengths were 9.50 ± 1.90 and 9.79 ± 1.94 in TAs and SPs, respectively. In tumor tissues, telomere lengths were 9.90 ± 1.93 and 9.99 ± 1.54 in TAs and SPs, respectively. These results were not significantly different (p = 0.500 and p = 0.824). To determine the correlation between telomere length and molecular genetic status, patients were divided into two groups according to the median value of telomere length.
Telomere length of normal mucosa in colorectal precancerous lesions
Molecular genetic analysis and telomere length were investigated in normal mucosa, and the results are presented in Table 1. Telomere shortening was observed in 32.8% (20/61) TAs and 31.3% (10/32) SPs, respectively. In TAs, telomere shortening was associated with PIK3CA amplification (81.3% vs. 18.8%, p < 0.001). KRAS mutation was inversely correlated with telomere shortening; however, it did not show statistical significance (13.3% vs. 86.7%, p = 0.11). SPs with BRAF mutation (66.7%) had shorter telomeres than those without BRAF mutation (23.1%), and this difference was of borderline statistical significance (p = 0.060). In SPs, telomere shortening was associated with PIK3CA amplification (55.6% vs. 21.7%), but it showed no statistical significance (p = 0.096). Other clinical and molecular characteristics were not associated with telomere length.
Table 1.
Characteristics of telomere length in normal tissues of TAs and SPs
| TA | SP | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Short (N, %) | Long (N, %) | p | Short (N, %) | Long (N, %) | p | |
| Total | 20 (32.8) | 41 (67.2) | 10 (31.3) | 22 (68.8) | ||
| Age | .507 | .681 | ||||
| < 65 | 13 (36.1) | 23 (63.9) | 7 (29.2) | 17 (70.8) | ||
| ≥ 65 | 7 (28.0) | 18 (72.0) | 3 (37.5) | 5 (62.5) | ||
| Sex | .797 | .355 | ||||
| Male | 13 (31.7) | 28 (68.3) | 8 (36.4) | 14 (63.3) | ||
| Female | 7 (35.0) | 13 (65.0) | 2 (20.0) | 8 (80.0) | ||
| Region | .382 | .648 | ||||
| Right | 4 (23.5) | 13 (76.5) | 3 (42.9) | 4 (57.1) | ||
| Left | 16 (36.4) | 28 (63.6) | 7 (28.0) | 18 (72.0) | ||
| KRAS mutation | .111 | 1.00 | ||||
| (+) | 2 (13.3) | 13 (86.7) | 0 (0) | 1 (100) | ||
| (-) | 18 (39.1) | 28(60.9) | 10 (32.3) | 21 (67.7) | ||
| BRAF mutation | .060 | |||||
| (+) | - | - | 4 (66.7) | 2 (33.3) | ||
| (-) | 20 (32.8) | 41 (67.2) | 6 (23.1) | 20 (76.9) | ||
| MSI | .384 | .572 | ||||
| (+) | 3 (50.0) | 3 (50.0) | 2 (50.0) | 2 (50.0) | ||
| (-) | 17 (30.9) | 38 (69.1) | 8 (28.6) | 20 (71.4) | ||
| mtMSI | 1.00 | 1.00 | ||||
| (+) | 4 (36.4) | 7 (63.6) | 2 (33.3) | 4 (66.7) | ||
| (-) | 16 (32.0) | 34 (68.0) | 8 (30.8) | 18 (69.2) | ||
| PIK3CA amplification | < .001 | .096 | ||||
| (+) | 13 (81.3) | 3 (18.8) | 5 (55.6) | 4 (44.4) | ||
| (-) | 7 (15.6) | 38 (84.4) | 5 (21.7) | 18 (78.3) | ||
Telomere length in colorectal precancerous lesions
Telomere shortening was found in 32.8% (20/61) TAs and 18.8% (6/32) SPs, without a significant difference (p = 0.152, Table 2). KRAS and BRAF mutations were mutually exclusive in TAs and SPs (p < 0.001). Telomere length was significantly short in TA tissues with mtMSI (63.6%) than in those without mtMSI (36.4%, p = 0.030). Other clinical and molecular characteristics were not associated with telomere length.
Table 2.
Characteristics of telomere length in tumor tissue of TAs and SPs
| TA | SP | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Short (N, %) | Long (N, %) | p | Short (N, %) | Long (N, %) | p | |
| Total | 20 (32.8) | 41 (67.2) | 6 (18.8) | 26 (81.3) | ||
| Age | .507 | .681 | ||||
| < 65 | 11 (30.6) | 25 (69.4) | 4 (16.7) | 20 (83.3) | ||
| ≥ 65 | 9 (36.0) | 16 (64.0) | 2 (25.0) | 6 (75.0) | ||
| Sex | .746 | 1.00 | ||||
| Male | 14 (34.1) | 27 (65.9) | 4 (18.2) | 18 (81.8) | ||
| Female | 6 (30.0) | 14 (70.0) | 2 (20.0) | 8 (80.0) | ||
| Region | .140 | .296 | ||||
| Right | 8 (47.1) | 9 (52.8) | 0 (0) | 7 (100) | ||
| Left | 12 (27.3) | 32 (72.7) | 6 (24.0) | 19 (76.0) | ||
| KRAS mutation | .959 | 1.00 | ||||
| (+) | 5 (33.3) | 10 (66.7) | (0) | 1 (100) | ||
| (-) | 15 (32.6) | 31 (67.4) | 6 (19.4) | 25 (80.6) | ||
| BRAF mutation | 1.00 | |||||
| (+) | - | - | 1 (16.7) | 5 (83.3) | ||
| (-) | 20 (32.8) | 41 (67.2) | 5 (19.2) | 21 (80.8) | ||
| MSI | 1.00 | 1.00 | ||||
| (+) | 2 (33.3) | 4 (66.7) | 1 (25.0) | 3 (75.0) | ||
| (-) | 18 (32.7) | 37 (67.3) | 5 (17.9) | 23 (82.1) | ||
| mtMSI | .030 | 1.00 | ||||
| (+) | 7 (63.6) | 4 (36.4) | 1 (16.7) | 5 (83.3) | ||
| (-) | 13 (26.0) | 37 (74.0) | 5 (19.2) | 21 (80.8) | ||
| PIK3CA amplification | .544 | .648 | ||||
| (+) | 4 (25.0) | 12 (75.0) | 1 (11.1) | 8 (88.9) | ||
| (-) | 16 (35.6) | 29 (64.4) | 5 (21.7) | 18 (78.3) | ||
Discussion
Previous studies have shown that telomere length is associated with the clinicopathological characters or survival rates in colorectal cancer (CRC) [7-9]. This cancer is considered a heterogeneous disease, as its precursors TA and SP, exhibit distinct pathological features and molecular signatures. To identify colorectal carcinogenesis and the potential markers for its prognosis, we used multitier genetic approaches with telomeres in both normal and tumor tissues of TAs and SPs. A previous study used real-time PCR to accurately identify telomere length in normal and tumor tissues [13]. This technique is the only practical high-throughput method available, especially in large-scale epidemiologic studies, therefore, we also examined telomere length by real-time PCR.
In this study, telomere length was found to be similar in TAs and SPs. Interestingly, telomere shortening in TAs was associated with PIK3CA amplification, whereas it was more frequent in SPs with BRAF mutation than in those without BRAF mutation. These results suggest that telomere status may play an important role in TA and SP developments in association with PIK3CA amplification and BRAF mutation, respectively. Although the precise mechanism of telomere regulation in colorectal lesions is still unclear, previous studies demonstrated that telomere shortening was observed as an early event in the process of colorectal carcinogenesis [10,16,17]. This indicated that telomere shortening in normal mucosa may additionally provide a background for rapid cancer progression through chromosome instability [10]. Therefore, telomere shortening has been linked to genetic changes like PIK3CA amplification and BRAF mutation, suggesting a pivotal role in the progression to TAs or SPs.
Moreover, mitochondrial MSI (mtMSI) was associated with telomere shortening in TAs. Our previous study showed that mtMSI was frequent in TAs as an early event and that TAs with mtMSI show an independent carcinogenesis pathway, resulting in poor prognosis [11]. Both mitochondria and telomeres are considered to be key instigators of natural ageing. Previous studies have reported that telomerase-deficient mice with severe telomere dysfunction showed disorders of mitochondrial biogenesis and decreased energy production directly linked to p53 [18-20]. Therefore, the telomere-p53-mitochondria axis may support our results showing that telomere-shortening induces mitochondrial instability in TAs. Taken together, we suggest that the disordered telomere-mitochondria axis in aging or genetic changes triggers colorectal carcinogenesis. Though the correlation between CRC prognosis and telomere length is still controversial, this possible linkage should be considered further with multifactorial gene analysis.
In conclusion, we identified that telomere shortening in normal tissues may contribute to either the tubular adenoma-carcinoma pathway or the serrated pathway in association with PIK3CA amplification and BRAF mutation, respectively. This phenomenon indicates that these two genetic mechanisms confer equivalent selective growth advantages via telomere shortening. Moreover, an association between telomere and mitochondria was shown in TAs, and this and previous results suggested that this pathway could be targeted for cancer prevention [21]. These data suggest a new paradigm for colorectal carcinogenesis (Figure 1); therefore, further studies on molecular mechanisms, including more clinical cases are needed to clarify this hypothesis.
Figure 1.
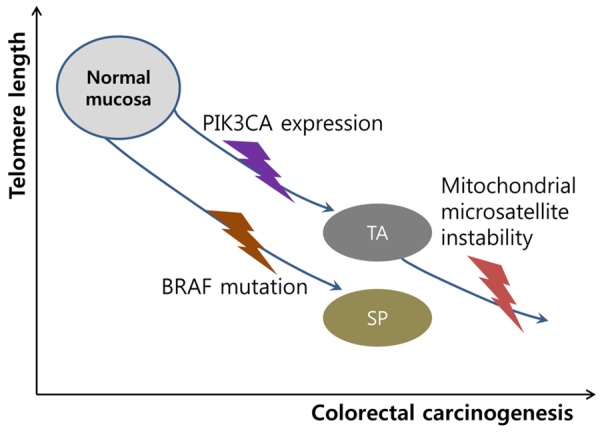
Schematic diagram of telomere shortening in colorectal carcinogenesis. In normal mucosa, telomere shortening induces colorectal carcinogenesis to tubular adenoma (TA) or serrated polyp (SP) dependently with PIK3CA or BRAF mutation, respectively. In TA, telomere shortening accelerates the progression via mitochondrial microsatellite instability.
Acknowledgements
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2014R1A6A3A04058057).
Disclosure of conflict of interest
None.
Abbreviations
- CRC
colorectal cancers
- MSI
microsatellite instability
- SP
serrated polyp
- TA
tubular adenoma
References
- 1.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 2.Forrester K, Almoguera C, Han K, Grizzle WE, Perucho M. Detection of high incidence of Kras oncogenes during human colon tumorigenesis. Nature. 1987;327:298–303. doi: 10.1038/327298a0. [DOI] [PubMed] [Google Scholar]
- 3.Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J. Clin. Oncol. 2005;23:609–618. doi: 10.1200/JCO.2005.01.086. [DOI] [PubMed] [Google Scholar]
- 4.Söreide K, Janssen EA, Söiland H, Körner H, Baak JP. Microsatellite instability in colorectal cancer. Br J Surg. 2006;93:395–406. doi: 10.1002/bjs.5328. [DOI] [PubMed] [Google Scholar]
- 5.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 6.Blackburn EH, Greider CW, Szostak JW. Telomeres and telomerase: the path from maize, tetrahymena and yeast to human cancer and aging. Nat Med. 2006;12:1133–1138. doi: 10.1038/nm1006-1133. [DOI] [PubMed] [Google Scholar]
- 7.Gertler R, Rosenberg R, Stricker D, Friederichs J, Hoos A, Werner M, Ulm K, Holzmann B, Nekarda H, Siewert JR. Telomere length and human telomerase reverse transcriptase expression as markers for progression and prognosis of colorectal carcinoma. J. Clin. Oncol. 2004;22:1807–1814. doi: 10.1200/JCO.2004.09.160. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Aranda C, de Juan C, Diaz-Lopez A, Sanchez-Pernaute A, Torres AJ, Diaz-Rubio E, Balibrea JL, Benito M, Iniesta P. Correlations of telomere length, telomerase activity, and telomeric-repeat binding factor 1 expression in colorectal carcinoma. Cancer. 2006;106:541–551. doi: 10.1002/cncr.21625. [DOI] [PubMed] [Google Scholar]
- 9.Rampazzo E, Bertorelle R, Serra L, Terrin L, Candiotto C, Pucciarelli S, Del Bianco P, Nitti D, De Rossi A. Relationship between telomere shortening, genetic instability, and site of tumour origin in colorectal cancers. Br J Cancer. 2010;102:1300–1305. doi: 10.1038/sj.bjc.6605644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suraweera N, Mouradov D, Li S, Jorissen RN, Hampson D, Ghosh A, Sengupta N, Thaha M, Ahmed S, Kirwan M, Aleva F, Propper D, Feakins RM, Vulliamy T, Elwood NJ, Tian P, Ward RL, Hawkins NJ, Xu ZZ, Molloy PL, Jones IT, Croxford M, Gibbs P, Silver A, Sieber OM. Relative telomere lengths in tumor and normal mucosa are related to disease progression and chromosome instability profiles in colorectal cancer. Oncotarget. 2016;7:36474–36488. doi: 10.18632/oncotarget.9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee JH, Hwang I, Kang YN, Choi IJ, Kim DK. Genetic characteristics of mitochondrial DNA was associated with colorectal carcinogenesis and its prognosis. PLoS One. 2015;10:e0118612. doi: 10.1371/journal.pone.0118612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee H, Lee JH, Kim DK, Choi IJ, Hwang I, Kang YN, Kim S. PIK3CA amplification is common in left side-tubular adenomas but uncommon sessile serrated adenomas exclusively with KRAS mutation. Int J Med Sci. 2015;12:349–353. doi: 10.7150/ijms.11281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cawthon RM. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res. 2009;37:e21. doi: 10.1093/nar/gkn1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valls C, Pinol C, Rene JM, Buenestado J, Vinas J. Telomere length is a prognostic factor for overall survival in colorectal cancer. Colorectal Dis. 2011;13:1265–1272. doi: 10.1111/j.1463-1318.2010.02433.x. [DOI] [PubMed] [Google Scholar]
- 15.Boardman LA, Johnson RA, Viker KB, Hafner KA, Jenkins RB, Riegert-Johnson DL, Smyrk TC, Litzelman K, Seo S, Gangnon RE, Engelman CD, Rider DN, Vanderboom RJ, Thibodeau SN, Petersen GM, Skinner HG. Correlation of chromosomal instability, telomere length and telomere maintenance in microsatellite stable rectal cancer: a molecular subclass of rectal cancer. PLoS One. 2013;8:e80015. doi: 10.1371/journal.pone.0080015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim HR, Kim YJ, Kim HJ, Kim SK, Lee JH. Telomere length changes in colorectal cancers and polyps. J Korean Med Sci. 2002;17:360–365. doi: 10.3346/jkms.2002.17.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roger L, Jones RE, Heppel NH, Williams GT, Sampson JR, Baird DM. Extensive telomere erosion in the initiation of colorectal adenomas and its association with chromosomal instability. J Natl Cancer Inst. 2013;105:1202–1211. doi: 10.1093/jnci/djt191. [DOI] [PubMed] [Google Scholar]
- 18.Sahin E, Colla S, Liesa M, Moslehi J, Müller FL, Guo M, Cooper M, Kotton D, Fabian AJ, Walkey C, Maser RS, Tonon G, Foerster F, Xiong R, Wang YA, Shukla SA, Jaskelioff M, Martin ES, Heffernan TP, Protopopov A, Ivanova E, Mahoney JE, Kost-Alimova M, Perry SR, Bronson R, Liao R, Mulligan R, Shirihai OS, Chin L, De-Pinho RA. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature. 2011;470:359–365. doi: 10.1038/nature09787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong KK, Maser RS, Bachoo RM, Menon J, Carrasco DR, Gu Y, Alt FW, DePinho RA. Telomere dysfunction and Atm deficiency compromises organ homeostasis and accelerates ageing. Nature. 2003;421:643–648. doi: 10.1038/nature01385. [DOI] [PubMed] [Google Scholar]
- 20.Hu J, Hwang SS, Liesa M, Gan B, Sahin E, Jaskelioff M, Ding Z, Ying H, Boutin AT, Zhang H, Johnson S, Ivanova E, Kost-Alimova M, Protopopov A, Wang YA, Shirihai OS, Chin L, De-Pinho RA. Antitelomerase therapy provokes ALT and mitochondrial adaptive mechanisms in cancer. Cell. 2012;148:651–663. doi: 10.1016/j.cell.2011.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee H, Cho JH, Park WJ, Jung SJ, Choi IJ, Lee JH. Loss of the association between telomere length and mitochondrial DNA copy number contribute to colorectal carcinogenesis. Pathol Oncol Res. 2017 doi: 10.1007/s12253-017-0245-z. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]


