Figure 2.
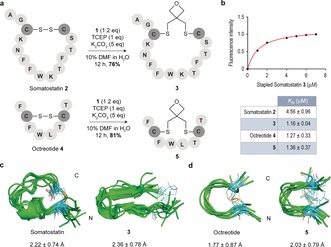
a) Stapling of disulfide‐containing cyclic peptides 2 and 4. b) Binding affinity studies. K D values were determined by tryptophan fluorescence spectroscopy. c),d) Structural ensembles obtained by 0.5 μs MD simulations. The peptide backbone is in green. Carbon atoms of Cys residues as well as of the oxetane moiety are in cyan. The numbers indicate the root‐mean‐square deviation (RMSD) for heavy‐atom superimposition of the backbone with respect to the average structure.
