Figure 1.
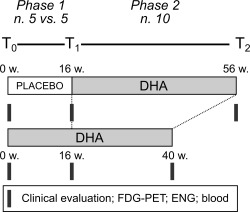
Study design. We conducted a 2‐phase trial consisting of a randomized double‐blind placebo‐controlled phase of 16 weeks, and an open‐label phase of 40‐week docosahexaenoic acid (DHA) supplementation. White bar = placebo treatment; gray bars = DHA treatment. Black blocks indicate the time points of clinical assessment (brain 18‐fluorodeoxyglucose positron emission tomography [FDG‐PET], electromyography/electroneurography [ENG], and blood sampling [blood]). w = weeks.
