Figure 2.
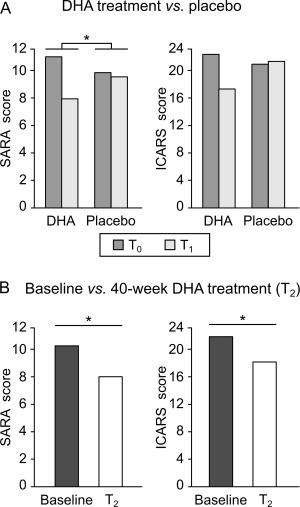
Clinical assessment in the double‐blind randomized placebo‐controlled phase and the open‐label phase. (A) Scale for the Assessment and Rating of Ataxia (SARA) and International Cooperative Ataxia Rating Scale (ICARS) scores in the docosahexaenoic acid (DHA)‐treated group and in the placebo‐treated group before (T0, dark gray bars) and after 16‐week DHA/placebo treatment (T1, light gray bars) in spinocerebellar ataxia 38 (SCA38) patients. (B) SARA scores and ICARS scores at baseline (dark bars) and after 40‐week DHA treatment (T2, white bars) in SCA38 patients. *p < 0.05.
