Abstract
Many women consider botanical dietary supplements (BDSs) as safe alternatives to hormone therapy for menopausal symptoms. However, the effect of BDSs on breast cancer risk is largely unknown. In the estrogen chemical carcinogenesis pathway, P450 1B1 metabolizes estrogens to 4-hydroxylated catechols, which are oxidized to genotoxic quinones that initiate and promote breast cancer. In contrast, P450 1A1 catalyzed 2-hydroxylation represents a detoxification pathway. The current study evaluated the effects of red clover, a popular BDS used for women’s health, and its isoflavones, biochanin A (BA), formononetin (FN), genistein (GN), and daidzein (DZ), on estrogen metabolism. The methoxy estrogen metabolites (2-MeOE1, 4-MeOE1) were measured by LC-MS/MS, and CYP1A1 and CYP1B1 gene expression was analyzed by qPCR. Nonmalignant ER-negative breast epithelial cells (MCF-10A) and ER-positive breast cancer cells (MCF-7) were derived from normal breast epithelial tissue and ER+ breast cancer tissue. Red clover extract (RCE, 10 μg/mL) and isoflavones had no effect on estrogen metabolism in MCF-10A cells. However, in MCF-7 cells, RCE treatments downregulated CYP1A1 expression and enhanced genotoxic metabolism (4-MeOE1/CYP1B1 > 2-MeOE1/CYP1A1). Experiments with the isoflavones showed that the AhR agonists (BA, FN) preferentially induced CYP1B1 expression as well as 4-MeOE1. In contrast, the ER agonists (GN, DZ) downregulated CYP1A1 expression likely through an epigenetic mechanism. Finally, the ER antagonist ICI 182,780 potentiated isoflavone-induced XRE-luciferase reporter activity and reversed GN and DZ induced downregulation of CYP1A1 expression. Overall, these studies show that red clover and its isoflavones have differential effects on estrogen metabolism in “normal” vs breast cancer cells. In breast cancer cells, the AhR agonists stimulate genotoxic metabolism, and the ER agonists downregulate the detoxification pathway. These data may suggest that especially breast cancer patients should avoid red clover and isoflavone based BDSs when making choices for menopausal symptom relief.
Introduction
Breast cancer remains the most prevalent cancer among women, with an estimated quarter of a million breast cancer diagnoses in 2015 alone.1 Estrogens can initiate cancer when their binding to the estrogen receptor (ER) leads to increased cell proliferation and the likelihood of DNA mutations (hormonal pathway of carcinogenesis).2 Breast cancer risk is also influenced by estrogen metabolism, and the genotoxic estrogen quinones formed in this process3−5 can be modulated by dietary means, including botanical dietary supplements (BDSs).2,6,7 As traditional hormone therapy (HT) is associated with an increased risk of breast cancer, many women use BDSs, which are perceived as safer alternatives for the relief of menopausal symptoms.2,8−10 However, efficacy claims are not only disallowed for BDSs but also remain questionable for botanicals used in this field. In particular, the effect of estrogenic BDSs on estrogen metabolism is unknown.
Ligand activation of the aryl hydrocarbon receptor (AhR) followed by cooperative binding of AhR and the aryl hydrocarbon receptor nuclear translocator to xenobiotic response elements (XREs) upregulate P450 1B1,11 which metabolizes estrogens to 4-hydroxylated catechols (Scheme 1). These catechols are oxidized to genotoxic, unstable quinones that form depurinating adducts and apurinic sites, collectively resulting in carcinogenesis via the estrogen chemical carcinogenesis pathway (Scheme 1).4,12 Given that 4-OHE2 transforms estrogen receptor-negative (ER−) cells into a malignant phenotype,13−15 activation of the chemical pathway is likely an important event in breast cancer initiation and/or promotion. AhR signaling also activates P450 1A1, which metabolizes estrogens to 2-hydroxylated metabolites (Scheme 1).16−18 However, the 2-hydroxylation pathway is negatively correlated with breast cancer risk3 because the 2-hydroxylated catechol estrogens reduce E2-induced proliferation19 and 2-methoxyestradiol, formed from metabolism of the catechol by catechol-O-methyltransferase (COMT), inhibits E2-induced proliferation of breast cancer cells.20 Thus, the 2-hydroxylation pathway can be regarded as a detoxification pathway (Scheme 1). The lack of information on the modulation of estrogen metabolism by botanicals used for menopausal symptom relief motivated the present study.
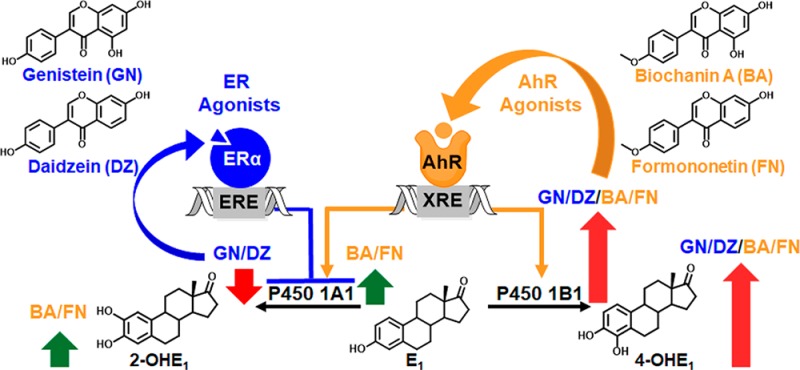
Scheme 1. AhR and ER Agonists from Red Clover Modulate Estrogen Chemical Carcinogenesis.
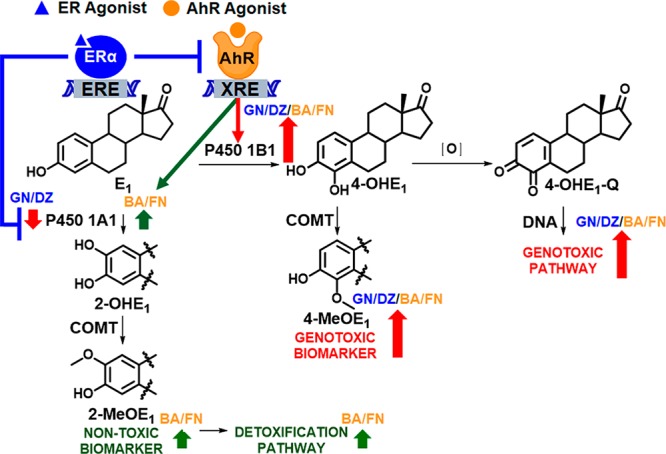
AhR agonists induce AhR binding to xenobiotic response elements (XREs) in target genes, CYP1A1 and CYP1B1. Modulation of CYP1A1 and/or CYP1B1 expression in turn alters levels of P450 1A1 and P450 1B1 enzymes that metabolize estrogens (E1) to non-toxic (2-OHE1) and genotoxic metabolites (4-OHE1). Oxidation of 4-OHE1 generates genotoxic estrogen quinones (4-OHE1-Q). Alternatively, catechol-O-methyltransferase (COMT) converts 2-OHE1 and 4-OHE1 to 2-MeOE1 and 4-MeOE1, which have been used as non-toxic and genotoxic biomarkers, respectively, in this study.2,6 As indicated with red and green arrows, AhR agonists preferentially increase the genotoxic pathway and ER agonists repress AhR activation and downregulate CYP1A1 expression.
Several popular BDSs used for menopausal symptom management contain constituents that alter P450 1A1 and P450 1B1 gene expression (i.e., CYP1A1 and CYP1B1) or inhibit these enzymes in various cell lines.21,22 However, studies investigating the broader effect of these BDSs on estrogen metabolism, specifically “normal” vs cancerous mammary epithelial cells, are scarce. Previous studies reported that two popular and relevant BDSs, licorice and hops, can modulate estrogen metabolism in breast cells. Evidence was built on the LC-MS/MS detection of the methoxy estrogen metabolites 2-MeOE1 (non-toxic biomarker) and 4-MeOE1 (genotoxic biomarker, Scheme 1).2,6 These studies found that one pharmacopoeial licorice species, Glycyrrhiza inflata, and its marker compound, licochalcone A (AhR antagonist), decreased 4-MeOE1 and CYP1B1 expression in the nonmalignant ER– breast epithelial cell line MCF-10A.6 Furthermore, in both MCF-10A cells and the ER+ breast cancer cell line MCF-7, hops and its bioactive constituent, 6-prenylnaringenin (an AhR agonist), preferentially induced the 2-hydroxylation detoxification pathway (2-MeOE1 > 4-MeOE1).2 Phytoestrogens, such as genistein (GN), daidzein (DZ), liquiritigenin, and S-equol, reportedly modulated CYP1A1 and CYP1B1 expression through both ERα and AhR mechanisms in ER+ MCF-7 cells.23 Hence, in ER+ breast tissue, botanical constituents can target both ERα and AhR and significantly alter CYP1A1 and/or CYP1B1 expression (Scheme 1). One key insight from these previous studies is that the impact of BDSs used for women’s health on estrogen metabolism in breast cells should be studied systematically in different models, for example, ER+ and ER– phenotypes, as outcomes vary according to cell/tissue type.
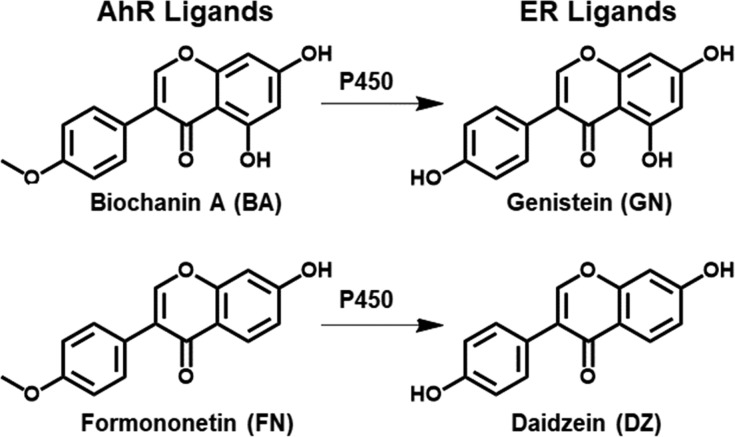
The current study uses MCF-10A and MCF-7 cells, which were derived from normal breast epithelium and ER+ tumors, to determine the effects of a red clover extract (RCE) and four of its bioactive marker isoflavones, GN, DZ, biochanin A (BA), and formononetin (FN), on estrogen metabolism. The dried flowering above ground parts of red clover (Trifolium pratense L., Fabaceae) have been used traditionally as an expectorant and against skin inflammation;24 however, due to its estrogenic isoflavone content, the predominant current use of the extract is for menopausal symptoms.25,26 GN and DZ (Figure 1) are estrogenic isoflavones from red clover and soy that slightly prefer ERβ over ERα.27 BA and FN are the 4′-methoxy ether analogues of GN and DZ that undergo P450 catalyzed O-demethylation to GN and DZ in vivo (Figure 1).2,28,29 In addition, BA and FN are AhR agonists, which can induce P450 metabolism;30−34 however, to date no studies have focused on their effect on estrogen metabolites in breast cells. The current study fills this gap by evaluating the effect of chemically standardized RCE and marker isoflavones on estrogen metabolites and CYP1A1 and CYP1B1 expression in both MCF-10A and MCF-7 cells. The goal of this study was to better understand the impact of red clover dietary supplements on the alteration of estrogen metabolism.
Figure 1.
Key bioactive isoflavones in red clover.
Materials and Methods
Chemicals and Extracts
The RCE used in this study was an autohydrolyzed hydroalcoholic extract of the aerial parts of Trifolium pratense L., Fabaceae, which had previously been used in a clinical trial.25 The extract contained 30% w/w isoflavones [BA (14.47%), FN (14.26%), GN (0.41%), and DZ (0.23%)] and was manufactured by Pure World Botanicals, Inc. (South Hackensack, NJ) as described previously.25,27 The chemical profile of this clinical red clover extract, batch BC190, which has been used throughout these studies, was extensively described previously.35 All pure compounds were obtained from Sigma-Aldrich (St. Louis, MO), unless otherwise indicated.
Cell Lines and Culture Conditions
HC-04 and MCF-10A cells were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). MCF-7 WS8 cells, provided by Dr. C. Jordan, are an estrogen sensitive cell line (ER+) and were cloned from MCF-7 cells, as previously described.36 MCF-7 WS8 cells were maintained in RPMI 1640 media supplemented with 10% fetal bovine serum, 1% glutaMAX, 1% AB/AM, 1% nonessential amino acids, and insulin (6 ng/mL). MCF-10A cells were cultured in DMEM/F12 supplemented with epidermal growth factor (20 ng/mL), cholera toxin (100 ng/mL), hydrocortisone (0.5 μg/mL), insulin (10 μg/mL), 5% horse serum, and 1% penicillin–streptomycin. HC-04 cells, which are nearly identical to HepG2 cells (BEI Resources, Manassas, VA and ATCC database), were maintained in DMEM/F12 with 10% FBS and 1% penicillin–streptomycin. All cell lines were authenticated via determination of the short tandem repeat (STR) profile using an ABI 3730xl DNA analyzer and the Promega GenPrint 10 system (Promega, Madison, WI, USA) and GeneMapper 5.0 analysis software (Thermo Fisher Scientific, Waltham, MA, USA). HC-04 cells and MCF-10A cells were in 100% agreement with the STR profile according to the ATCC database. The MCF-7 WS8 cells were in 93% agreement with the MCF-7 cells from ATCC; however, they showed one allele deletion (D5S818:12), indicating a slight difference between the MCF-7 WS8 subclone cell line and the MCF-7 ATCC cell line.36
Analysis of Estrogen Metabolism by LC-MS/MS
After treating MCF-10A and MCF-7 cells with E2, rapid conversion to E1 occurs. Thus, the methoxyestrone metabolites 2-MeOE1 and 4-MeOE1 were measured by LC-MS/MS as indicators of the level of estrogen 2-hydroxylation and the 4-hydroxylation pathways as previously described with modifications.2,6 Metabolite standards were obtained from Steraloids Inc. (Newport, RI). The internal standard 4-MeOE1-1,4,16,16-d4 was obtained from CDN Isotope (Pointe-Claire, Quebec). In previous studies, the LC-MS/MS method cotreated compounds/extracts with E2 for 48 h.2,6 When applying this method, RCE treatment yielded several LC-MS peaks that interfered with the 2-MeOE1 and 4-MeOE1 peaks. To eliminate this issue, a PBS washing step was added after treatment with RCE/isoflavones (48 h) and before incubation with E2 alone (1 μM for 24 h). In this modified method, cells were cultured for 72 h and MCF-7 cells were plated in 6-well plates in RPMI 1640 media without phenol red and supplemented with 10% charcoal-stripped FBS, 1% glutaMAX, 1% AB/AM, 1% nonessential amino acids, and insulin (6 ng/mL) at 3.5 × 105 cells/well. MCF-10A cells (1.6 × 105 cells/well) were plated in 6-well plates in DMEM/F12 media without phenol red and supplemented with 5% charcoal-stripped horse serum, epidermal growth factor (20 ng/mL), cholera toxin (100 ng/mL), hydrocortisone (0.5 μg/mL), insulin (10 μg/mL), and 1% penicillin–streptomycin. After 24 h, cells were incubated for 48 h with RCE/isoflavones. Cells were washed with PBS and then incubated with 1 μM E2 for 24 h. Cell media were collected and spiked with 0.4 nM internal standard (4-MeOE1-d4) and 2 mM ascorbic acid. The media were then extracted with dichloromethane (2 × 4 mL). The combined organic layers were then dried under nitrogen. Dansylation was performed as described previously2,6 with NaHCO3 buffer (75 μL, 0.1 M, pH 9.5) and 75 μL of dansyl chloride in acetone (1.25 mg/mL). Derivatized samples were analyzed by positive ion electrospray tandem mass spectrometry using an Agilent 1200 series nano flow LC system (Agilent Technologies, Santa Clara, CA) coupled to an AB SCIEX QTRAP 5500 system (AB SCIEX, Framingham, MA) as described previously.2,6 Quantitation was performed using Analyst software (Applied Biosystems, Forster City, CA), and data were normalized to the DMSO treatment.
Analysis of Gene Expression by RT-qPCR
The Ambion Cells to Ct kit for RT-qPCR was obtained from Thermo Fisher Scientific (Waltham, MA), and the experiments were performed according to the manufacturer’s protocol. In 96-well plates, MCF-7 cells were plated in RPMI 1640 media without phenol red and supplemented with 10% charcoal-stripped FBS, 1% glutaMAX, 1% AB/AM, 1% nonessential amino acids, and insulin (6 ng/mL). MCF-10A cells were plated in DMEM/F12 media without phenol red and supplemented with 5% charcoal-stripped horse serum, epidermal growth factor (20 ng/mL), cholera toxin (100 ng/mL), hydrocortisone (0.5 μg/mL), insulin (10 μg/mL), and 1% penicillin–streptomycin 24 h before treatment with RCE/isoflavones. Cells were incubated an additional 24 h with treatments before lysis. Using an Applied Biosystems StepOnePlus real-time PCR system (Thermo Fisher Scientific, Waltham, MA), RT-qPCR was performed with lysates (2 μL), TaqMan 1-step RT-PCR master mix, and CYP1A1 and CYP1B1 primers with FAM-MGB probe or an HPRT1 primer with VIC-MGB probe. Data were analyzed with the comparative CT method (ΔΔCT) and expressed as fold induction relative to DMSO as the negative control.
XRE-Luciferase Reporter Assay
In 12-well plates, HC-04 cells were plated in DMEM/F12 media with 10% FBS and 1% penicillin–streptomycin and MCF-7 cells were plated in RPMI 1640 media without phenol red and supplemented with 10% charcoal-stripped FBS, 1% glutaMAX, 1% AB/AM, 1% nonessential amino acids, and insulin (6 ng/mL) overnight. Cells were transfected at 70% confluency with luciferase and renilla plasmids (Promega, Madison, WI), XRE pGL4.43, and pRL-TK, respectively, using Lipofectamine 2000 reagent for 6 h. Cells were treated with the RCE/isoflavones for 24 h and lysed with buffer. The lysates were analyzed for luciferase activity according to Promega’s dual-luciferase reporter assay system protocol using BioTek (Winooski, VT) synergy H4 hybrid multi-mode microplate reader.6 For experiments with ICI 182,780 in MCF-7 cells, the cells were pretreated with ICI 182,780 for 2 h and incubated with compounds for an additional 24 h before the cells were lysed and analyzed for luciferase activity.
Statistical Analysis
The analyzed data were expressed as the mean ± SEM of three independent experiments. Significance was determined using one-way ANOVA with Dunnett’s multiple comparison post test, comparing test sample data to the control sample. P < 0.05 indicated significance (*). Statistical analysis of CYP1A1 and CYP1B1 expression and XRE-luciferase reporter activity in MCF-7 cells employed Student’s t test to compare samples with and without ICI 182,780.
Results
Red Clover’s Modulation of Oxidative Estrogen Metabolism and CYP1A1/CYP1B1 Expression in MCF-10A and MCF-7 Cells
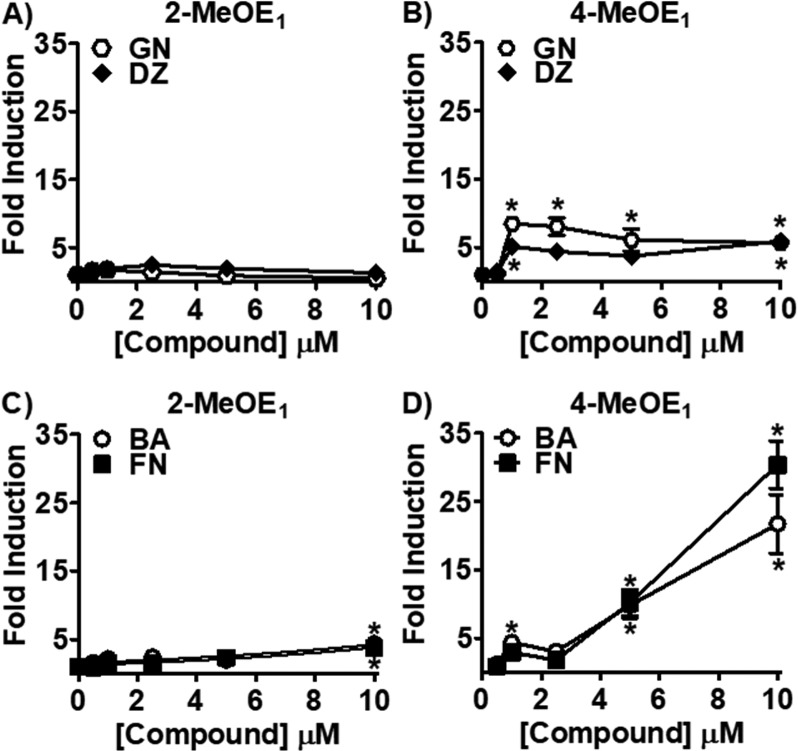
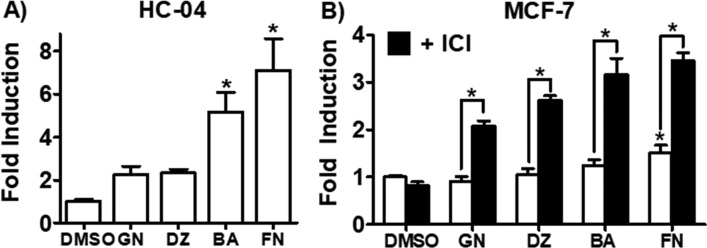
In order to analyze the effect of RCE on estrogen metabolism in normal epithelial breast cells, MCF-10A cells were used. The current study used an LC-MS/MS method to measure methoxyestrone metabolites that was slightly modified (see Materials and Methods).2 RCE (Figure 2A) and its major isoflavones (Figure S1), GN, DZ, BA, and FN, showed no effect. These data suggested that in normal breast epithelial cells RCE has no effect on the chemical estrogen carcinogenesis pathway. To investigate whether RCE and/or its isoflavones modulate estrogen metabolism in ER+ breast cancer cells, the same experiments performed in MCF-10A cells were carried out in MCF-7 cells. Interestingly, here RCE (10 μg/mL) caused an increase in both estrogen 2-hydroxylation and 4-hydroxylation (Figure 2A). Levels of 4-MeOE1 reached 10-fold induction and represented nearly double of the 2-MeOE1 levels observed after a 2 day treatment with RCE (10 μg/mL). Similarly, CYP1B1 expression (5-fold) was twice that of CYP1A1 expression after 24 h of RCE treatment (10 μg/mL; Figure 2B). Interestingly, at lower concentrations (<5 μg/mL), RCE significantly downregulated CYP1A1 expression below basal levels (Figure 2B). As a result, E2 was predominately metabolized by P450 1B1 to potentially genotoxic catechols in MCF-7 cells under these conditions.
Figure 2.
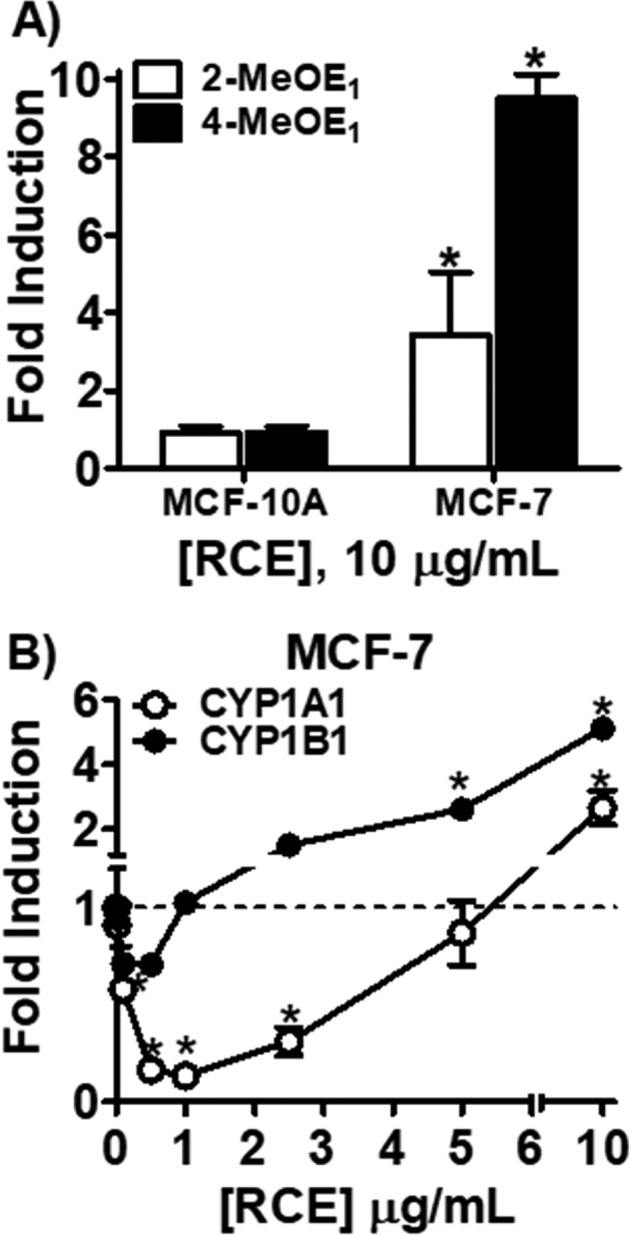
LC-MS/MS analysis of methoxyestrone metabolites (2-MeOE1, 4-MeOE1) in MCF-10A and MCF-7 cells and CYP1A1 and CYP1B1 expression levels in MCF-7 cells. (A) MCF-10A and MCF-7 cells were treated with RCE (10 μg/mL) and for 48 h, washed with PBS, and then treated for 24 h with E2 (1 μM). (B) CYP1A1 and CYP1B1 expression levels were determined after 24 h treatment with RCE in MCF-7 cells by RT-qPCR analysis.
Modulation of 2-MeOE1/4-MeOE1 by Isoflavones in MCF-7 Cells
To determine which isoflavones might be responsible for RCE’s stimulating effect on estrogen metabolism, MCF-7 cells were treated with different concentrations of GN, DZ, BA, and FN. GN and DZ had no significant effect on the 2-hydroxylation pathway (Figure 3A). Overall, GN and DZ at concentrations ranging from 1 to 10 μM increased the production of 4-MeOE1 by at least 5-fold (Figure 3B). However, this increase was not dose-dependent. In contrast, BA and FN (10 μM), increased 4-MeOE1 more than 2-MeOE1 metabolites, at least 8 and 5 times, respectively (Figure 3C,D). The maximum induction of 4-MeOE1 by BA and FN (10 μM) was 20- and 30-fold, respectively.
Figure 3.
LC-MS/MS analysis of methoxyestrone metabolites (2-MeOE1, 4-MeOE1) in MCF-7 cells after treatment with isoflavones. MCF-7 cells were treated for 48 h with the isoflavones, GN, DZ, BA, and FN, followed by 24 h treatment with E2 (1 μM). 2-MeOE1 and 4-MeOE1 metabolites were analyzed by LC-MS/MS. (A, B) Effect of the ER agonists, GN and DZ, on (A) 2-MeOE1 and (B) 4-MeOE1 metabolites. (C, D) Effect of the AhR agonists, BA and FN, on (C) 2-MeOE1 and (D) 4-MeOE1 metabolites.
Modulation of CYP1A1/CYP1B1 Expression by Isoflavones in MCF-7 Cells
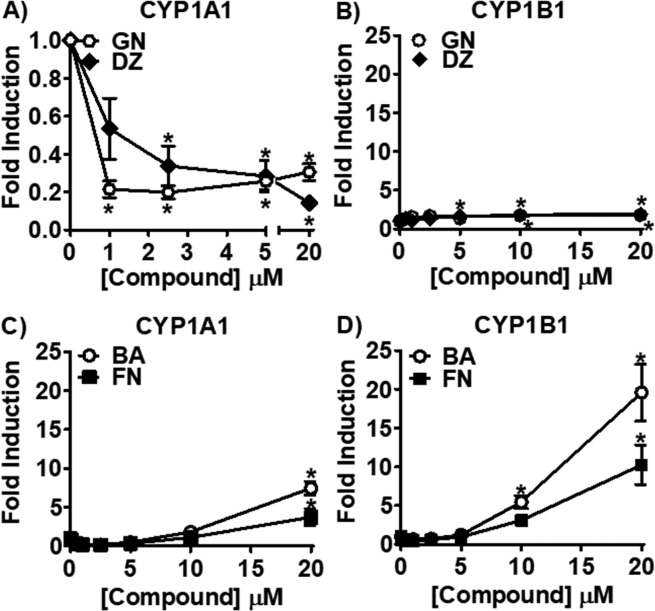
As Trifolium isoflavones increase the P450 1B1 catalyzed genotoxic pathway and have little to no effect on the P450 1A1 catalyzed detoxification pathway, their effect on CYP1A1 and CYP1B1 gene expression was measured to study these differential effects in more depth. GN and DZ dose-dependently downregulated CYP1A1 expression (Figure 4A). GN and DZ caused a small, but not dose-dependent, increase in CYP1B1 expression to 2-fold at the highest test concentration (20 μM, Figure 4B). In contrast, BA and FN increased both CYP1A1 and CYP1B1 dose-dependently. BA and FN upregulated CYP1A1 expression to 8- and 4-fold, respectively, at 20 μM (Figure 4C). Even larger induction was observed with CYP1B1 expression (Figure 4D).
Figure 4.
CYP1A1 and CYP1B1 expression levels analyzed by RT-qPCR in MCF-7 cells after treatment with isoflavones. MCF-7 cells were treated for 24 h with the isoflavones, GN, DZ, BA, and FN, and CYP1A1 and CYP1B1 expression was analyzed by RT-qPCR. (A, B) Effect of the ER agonists, GN and DZ, on (A) CYP1A1 and (B) CYP1B1 expression. (C, D) Effect of the AhR agonists, BA and FN, on (C) CYP1A1 and (D) CYP1B1 expression.
Influence of the ER Antagonist, ICI 182,780, on Isoflavone-Regulated CYP1A1/CYP1B1 Expression in MCF-7 Cells
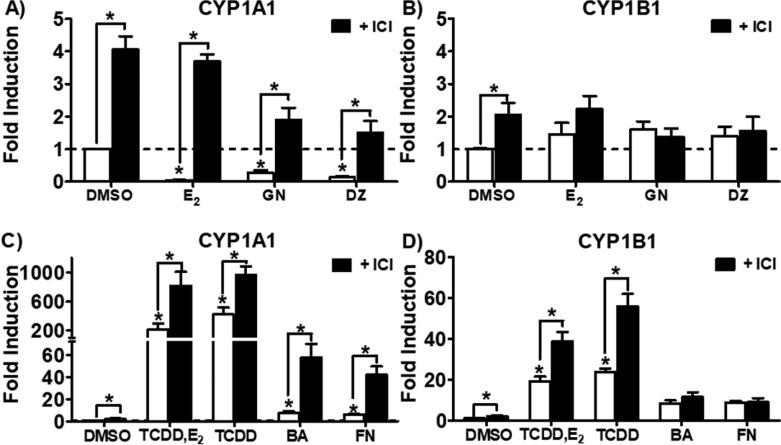
The role of ERα on CYP1A1 and CYP1B1 expression was investigated by treating MCF-7 cells with and without ICI 182,780 and the following treatments: ER agonists (E2, GN, and DZ), AhR agonists (TCDD, BA, and FN), or TCDD plus E2 (Figure 5). E2 (1 nM) alone significantly downregulated CYP1A1 expression (Figure 5A) and had no effect on CYP1B1 (Figure 5B). As expected, TCDD increased CYP1A1 expression (425-fold, Figure 5C) and CYP1B1 expression (28-fold, Figure 5D). TCDD-induced CYP1A1 expression (425-fold) was decreased to 220-fold by E2 (Figure 5C), but it had no effect on TCDD-induced CYP1B1 expression (Figure 5D). Compared to potent AhR agonists such as TCDD, BA, and FN, ICI 182,780 treatment alone modestly increased CYP1A1 (Figure 5A). ICI 182,780 and E2 treatments caused a similar and modest increase of CYP1B1 expression (Figure 5B). Similar to E2, pretreatment with ICI 182,780 followed by GN and DZ effectively eliminated CYP1A1 downregulation by these estrogenic isoflavones (Figure 5A). After pretreatment, ICI 182,780 was even more effective at potentiating BA and FN-mediated CYP1A1 upregulation, increasing it from 7- and 6-fold, respectively, to 55- and 40-fold (Figure 5C). In comparison with isoflavones alone, ICI 182,780 pretreatment did not significantly modulate CYP1B1 expression (Figure 5B, Figure 5D). When ICI was added to TCDD cotreated with E2 and TCDD treatment alone, both CYP1A1 (Figure 5C) and CYP1B1 fold induction (Figure 5D) greatly increased. These data suggest that ERα specifically influences the effect of isoflavones on CYP1A1 expression, without altering CYP1B1.
Figure 5.
CYP1A1 and CYP1B1 expression analyzed by RT-qPCR in MCF-7 cells after treatment with isoflavones. Cells were pretreated with ICI 182,780 (1 μM) for 2 h and ER agonists [E2 (1 nM), and GN and DZ (10 μM)] were added for an additional 24 h before RT-qPCR analysis of (A) CYP1A1 and (B) CYP1B1 levels. Treatments with AhR agonists [TCDD (10 nM), TCDD (10 nM) + E2 (1 nM), BA (10 μM) and FN (10 μM)] were added for an additional 24 h after 2 h ICI 182,780 pretreatment before RT-qPCR analysis of (C) CYP1A1 and (D) CYP1B1 levels.
XRE-Luciferase Reporter Activity in HC-04 and MCF-7 cells
To further elucidate mechanistic differences between the tested isoflavones and to compare AhR transcriptional activation by isoflavones, XRE-luciferase reporter activity was analyzed (Figures 6A and S2). HC-04 cells were transfected with an XRE-luciferase reporter plasmid and treated with isoflavones for 24 h. Both GN and DZ had no significant effect, while BA and FN significantly increased activity to 5- and 7-fold. XRE-luciferase activity was also measured in MCF-7 cells; however, only FN had a significant effect (Figure 6B). Because ERα can suppress AhR activation,23 the MCF-7 cells were pretreated with ICI 182,780 before isoflavone treatments and XRE-luciferase activity was determined. Interestingly, the XRE-luciferase fold induction by isoflavone treatment more than doubled with ICI 182,780 pretreatment (Figure 6B). ICI 182,780 alone had no effect on XRE-luciferase reporter activity. These data confirmed that BA and FN act as AhR agonists and that ERα downregulates XRE-reporter activity.
Figure 6.
XRE-luciferase reporter activity analyzed after 24 h treatment with isoflavones (10 μM), BA, FN, GN, and DZ, in (A) HC-04 cells and (B) in MCF-7 cells. ICI 182,780 (1 μM) was added 2 h before treatment with compounds, and cells were incubated for an additional 24 h before analysis of XRE-luciferase reporter activity.
Discussion
Although both P450 1A1 and P450 1B1 genes, CYP1A1 and CYP1B1, respectively, are coordinately upregulated by AhR agonists, ER agonists differentially regulate these genes.37 As many botanicals used for menopausal symptoms, like red clover, contain AhR agonists that can increase estrogen metabolism along with phytoestrogens38 that may downregulate CYP1A1 similarly to E2,37 evaluating their effect on estrogen metabolism in vitro is a means of enhancing the prediction of safety in vivo. Because the effect of RCE and its isoflavones on estrogen metabolism had not been previously investigated, we studied their effects in two different cell lines. MCF-10A cells are derived from normal human breast epithelial tissues. The ER expression level in the MCF-10A cell line is much lower than in the MCF-7 breast cancer cell line. Therefore, the MCF-10A cell line is considered ER–.39 Likewise, the levels of ER in normal breast tissues are often low.40 Hence, MCF-10A cells resemble normal breast epithelial tissues from healthy women more than MCF-7 cells, which are derived from ER+ breast tumors.
The study outcome showed that RCE did not modulate estrogen metabolism in “normal” ER- MCF-10A cells (Figure 2A). Although this study was limited to using this normal breast epithelial cell line, the conclusions regarding the safety of these supplements is consistent with the safety data from several clinical and in vivo studies using healthy women or animal models.25,41−43 An RCE (750 mg/kg/day) standardized to 15% isoflavones and given by gavage to ovariectomized rats did not increase proliferation in the breast.41 Available in the form of a commercial dietary supplement (Promensil), a red clover preparation was administered for 3 years to healthy women with a family history of breast cancer. In line with other evidence, Promensil did not increase breast density, which is known to increase the risk of breast cancer.42 Another study by Atkinson et al. found no difference in breast density or E2 levels.43 Furthermore, it appears that isoflavones may even decrease breast cancer risk.44 The association between breast cancer risk and the use of isoflavone supplements has also been analyzed among postmenopausal women. Using high content isoflavone supplements, at least 3 of the 28 total isoflavone dietary supplements included in the study, or any isoflavone supplement consumed for over 5 years were associated with a decreased risk in breast cancer.44 Red clover isoflavones possess other beneficial effects that may contribute to a positive safety profile in healthy women. The ERβ selectivity of GN and DZ may provide a better safety profile than classical estrogens.38,45 Additionally, GN inhibited DMBA-induced DNA oxidative damage and strand breaks in MCF-10A cells.46
However, other studies reported potential harmful effects of red clover phytoestrogens, such as stimulation of breast cancer cell proliferation and tumor growth at concentrations as low as 1 nM.47 Hsieh et al. showed that GN caused cell growth in MCF-7 cells, increased mammary gland growth in ovariectomized mice, and increased MCF-7 xenograft tumors.48 Downregulation of COMT mRNA and activity in MCF-7 cells has also been reported for phytoestrogens GN and DZ, which led to decrease in methylation of the genotoxic 4-OHE2 metabolite.49 In this study, MCF-7 breast cancer cells were also used to investigate red clover’s safety, and the results were compared to those from MCF-10A cells. It was noted that AhR responsiveness was much higher in MCF-7 cells than in MCF-10A cells. This has been observed when TCDD increased 2-MeOE1 and 4-MeOE1 levels in MCF-7 cells at least 4- and 7-fold more than in MCF-10A cells.39 Similarly, the current study (Figure S3) showed the difference between TCDD induced 2-MeOE1 and 4-MeOE1 levels to be 4- and 15-fold more in MCF-7 cells than MCF-10A cells, respectively. The same trend was observed for CYP1A1 and CYP1B1 expression (Figure S3). Subsequently, this study found that, while RCE caused an increase in overall estrogen oxidative metabolism, the P450 1B1-mediated genotoxic pathway was increased more than the P450 1A1 detoxification pathway in MCF-7 cells (Figure 2). Thus, RCE is predicted to be safe in normal breast tissues, yet it may modulate estrogen metabolism to increase genotoxic metabolites in breast cancer.
In MCF-7 cells, the individual effects of red clover isoflavones on estrogen metabolism in this study were determined as well. We previously determined that natural AhR agonists, such as 6-prenylnaringenin, increase estrogen metabolism through AhR activation.2,6 In the literature, BA and FN are reported as being AhR agonists,33,34 but whether GN and DZ activate AhR is controversial. Comparing relative AhR activation potencies, Bialesova et al. reported AhR activation by BA, FN, and GN (100 μM) to be 309-, 108-, and 27-fold, respectively, with DZ having no effect.30 A number of flavonoids and plant-derived indoles were tested in an assay to measure transcriptional activation of the AhR/ARNT dimer using an XRE reporter plasmid in yeast.50 The EC50s of BA and FN were 130 and 250 nM, respectively, designating them as being among the most potent compounds tested.50 EC50s were not reported for GN and DZ because the compounds were not potent enough for AhR transcriptional activation to be detected. However, GN and DZ (10 μM) significantly activated AhR in Hepa-1 cells to 12- and 14-fold, yet their induction of AhR activation did not reach 2-fold in MCF-7 cells.50 In the current study, GN and DZ (10 μM) increased XRE-luciferase reporter activity in HC-04 cells to 2-fold; however, the induction was 4 times lower than after BA and FN treatments (Figure 6A). AhR activation leading to CYP1A1 and CYP1B1 induction after isoflavone treatments has been reported,23,33,34 yet no studies have conducted a direct comparison of CYP1A1 and CYP1B1 induction by the four key marker isoflavones in MCF-7 cells. BA and FN (10 μM) reportedly increased CYP1A1 expression in MCF-7 cells after 6 h.33,34 BA (10 μM) doubled CYP1A1 expression and increased P450 1 specific activity 2.5-fold; however, CYP1B1 expression was not determined in this study. Studies aimed at determining the effect of GN and DZ on CYP1A1 and/or CYP1B1 are conflicting. Gong et al. produced results showing that GN and DZ (1 μM) increased both CYP1A1 and CYP1B1 expression to 5- and 3-fold, respectively, in MCF-7 cells after 4 h.23 However, Wagner et al. reported, after 24 h, a decrease in CYP1A1 with GN (1 μM) and DZ (10 μM) in estrogen sensitive MCF-7 cells (MCF-7 BUS); CYP1B1 was not measured in that study either.51 Overall, the present outcomes agree with other studies by showing that BA and FN were more potent than GN and DZ in activating CYP1A1 and CYP1B1. Even at the highest concentration tested, GN and DZ did not increase 2-MeOE1 metabolites (Figure 3A) or CYP1A1 (Figure 4A), and they only modestly increased CYP1B1 (2-fold, Figure 4B). As BA and FN significantly increased both P450 1A1 and P450 1B1 pathways, they are most likely the reason that RCE increased estrogen metabolism in MCF-7 cells.
On the other hand, GN and DZ, as ER agonists, are most likely the reason that RCE downregulated CYP1A1 at low concentrations in MCF-7 cells (Figure 2B) and demonstrated a relatively weak effect on estrogen 2-hydroxylation compared to 4-hydroxylation (Figure 2A). Estrogens limit AhR activation through various mechanisms, such as degradation of AhR or depletion of its available cofactors.52 Furthermore, when E2 activates ERα to displace AhR/ARNT, methylation on XREs in the CYP1A1 promoter region occurs.37 This mechanism specifically downregulates CYP1A1 without affecting CYP1B1 expression in MCF-7 cells.37 In the current study, E2 (1 nM) decreased basal and TCDD-induced CYP1A1 expression (Figure 5A,C). Moreover, Spink et al. reported that significant downregulation of TCDD-induced CYP1A1 expression/2-MeOE2 formation simultaneously occurred with upregulation of TCDD-induced CYP1B1/4-MeOE2 formation after E2 treatments (100 pM to 10 nM).53 Similarly, GN and DZ not only downregulated CYP1A1 but also increased CYP1B1/4-MeOE1 formation. However, it is likely but not certain that this increase in the 4-hydroxylation pathway by GN and DZ was mainly ERα mediated. GN and DZ are weak AhR agonists in MCF-7 cells,50 and studies regarding whether E2 modulates CYP1B1 expression are inconclusive.23,37,54,55 In the current study, E2 (1 nM) slightly, yet not significantly, increased basal CYP1B1 expression, but it produced no effect on TCDD-induced CYP1B1 expression (Figure 5D). Only a higher concentration of E2 (1 μM) increased CYP1B1 significantly (Figure S4), yet E2 (1 μM) did not have an effect on isoflavone modulation of CYP1B1 expression (Figure S5). Some literature reports have also shown that in the presence of E2, TCDD-induced CYP1B1 levels were not affected.37,55 However, Gong et al. observed that basal CYP1B1 levels were decreased,23 while others saw an increase in CYP1B1 expression with E2 treatment.54,55 Increased CYP1B1 expression by E2 may occur through an estrogen response element (ERE) located in the promoter region of the CYP1B1 gene.54 Thus, upregulation of CYP1B1 by GN and DZ could occur through ER and/or AhR mechanism(s) to increase the 4-hydroxylation of estrogens, yet downregulation of CYP1A1 by GN and DZ through an ERα-mediated mechanism was most likely the reason that RCE reduced CYP1A1 expression and thus only weakly induced 2-hydroxylation compared to 4-hydroxylation in MCF-7 cells.
It is probable that experimental conditions or cell type/context influenced the effect of estrogenic compounds on AhR activation. For instance, the concentration of E2 in media, length of E2 deprivation,53 and the well-documented differences in MCF-7 cell lines56−58 can lead to differences in ER levels that cause modulation in AhR responsiveness. To prove that the effect of these estrogens on AhR activation depends on cell context, several studies used ICI 182,780 to eliminate the effects of ERα. Similar to our studies, Gong et al. showed that ICI 182,780 and siERα increased basal levels of both CYP1A1 and CYP1B1, suggesting that even unliganded ERα suppressed constitutive AhR activation. ICI 182,780 also reversed downregulation of CYP1A1 by 10 nM23 and 100 pM concentrations of E2.51 Gong et al. also showed that siERα potentiated GN or DZ (1 μM) induced CYP1A1 and CYP1B1 expression in MCF-7 cells; ICI 182,780 (1 μM) did not have a significant effect on either gene.23 However, similar to this study, ICI 182,780 did reverse GN (1 μM) and DZ (10 μM) downregulation of CYP1A1 expression in MCF-7 BUS cells51 and TCDD-induced CYP1A1 expression.37 In the current study, ICI 182,780 (1 μM) potentiated TCDD-induced CYP1A1 and CYP1B1 expression (Figure 5C,D) and also increased CYP1A1 expression after isoflavone treatments (Figure 5A,C). Additionally, after MCF-7 cells were pretreated with ICI 182,780, all isoflavones significantly induced XRE-luciferase activity in MCF-7 cells (Figure 6B). No significant induction in XRE-luciferase reporter activity was observed with the isoflavones alone in MCF-7 cells (Figure 6B), in which XRE reporter activity is usually lower compared to other cell lines, such as HepG2 and Hepa-1 cells.2,50Figure S2 demonstrates that TCDD-induced XRE-luciferase reporter activity in HC-04 cells was 10-fold higher than in MCF-7 cells.
In conclusion, the study showed that ERα agonists suppressed AhR activation in MCF-7 cells. An epigenetic mechanism specifically targeting CYP1A1 was most likely the reason that RCE decreased 2-hydroxylation (CYP1A1), which led to a greater increase in the 4-hydroxylation pathway in MCF-7 cells. Although BA and FN increased estrogen metabolism, it is important to note that they are not bioavailable and are rapidly metabolized to GN and DZ in vivo.38,59,60 The effects of these phytoestrogens on estrogen metabolism are notable, especially because the 2-hydroxylation pathway is strongly associated with a decrease in breast cancer risk.3 Modulation of this benign pathway may vary in women based on several factors such as ER status. Furthermore, as women are turning to BDSs for relief of menopausal symptoms during breast cancer treatments (i.e., tamoxifen, aromatase inhibitors),61 it could be necessary to advise women with cancer against the consumption of certain BDSs containing isoflavones that induce the undesirable estrogen 4-hydroxylation pathway. Considering the in vitro nature of the available evidence, clinical studies are warranted that evaluate the corresponding safety parameters in these populations versus healthy women before recommendations about the clinical safety and population specificity of these BDSs can be made.
Acknowledgments
The authors sincerely thank Dr. Dejan Nikolić for his advice with LC-MS/MS experiments.
Glossary
Abbreviations
- AhR
aryl hydrocarbon receptor
- BA
biochanin A
- BDS
botanical dietary supplement
- COMT
catechol O-methyl transferase
- DZ
daidzein
- E1
estrone
- E2
estradiol
- ER
estrogen receptor
- FN
formononetin
- GN
genistein
- HT
hormone therapy
- 2-OHE1
2-hydroxyestrone
- 4-OHE1
4-hydroxyestrone
- 2-MeOE1
2-methoxyestrone
- 4-MeOE1
4-methoxyestrone
- P450 1A1
cytochrome P450 enzyme 1A1
- P450 1B1
cytochrome P450 enzyme 1B1
- RCE
red clover extract
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
- XRE
xenobiotic response element
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.chemrestox.7b00237.
Analysis of 2- and 4-MeOE1 levels after treating MCF-10A cells with RCE and isoflavones, TCDD-induced CYP1A1 and CYP1B1 expression and 2- and 4-MeOE1 levels in MCF-10A and MCF-7 cells, TCDD-induced XRE-luciferase reporter activity in HC-04 and MCF-7 cells, E2 induced CYP1A1/CYP1B1 expression levels, CYP1A1/CYP1B1 expression levels after cotreatment of MCF-7 cells with E2 and isoflavones (PDF)
Author Contributions
† T.L.D. and C.E.H. contributed equally to this work.
This work was supported through grant P50 AT000155 by NCCIH and ODS to the UIC/NIH Center for Botanical Dietary Supplements Research. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Siegel R. L.; Miller K. D.; Jemal A. (2016) Cancer statistics, 2016. Ca-Cancer J. Clin. 66, 7–30. 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- Wang S.; Dunlap T. L.; Howell C. E.; Mbachu O. C.; Rue E. A.; Phansalkar R.; Chen S. N.; Pauli G. F.; Dietz B. M.; Bolton J. L. (2016) Hop (Humulus lupulus L.) extract and 6-prenylnaringenin induce P450 1A1 catalyzed estrogen 2-hydroxylation. Chem. Res. Toxicol. 29, 1142–1150. 10.1021/acs.chemrestox.6b00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson J. N.; Falk R. T.; Schairer C.; Moore S. C.; Fuhrman B. J.; Dallal C. M.; Bauer D. C.; Dorgan J. F.; Shu X. O.; Zheng W.; Brinton L. A.; Gail M. H.; Ziegler R. G.; Xu X.; Hoover R. N.; Gierach G. L. (2017) Association of estrogen metabolism with breast cancer risk in different cohorts of postmenopausal women. Cancer Res. 77, 918–925. 10.1158/0008-5472.CAN-16-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton J. L.; Dunlap T. L. (2017) Formation and biological targets of quinones: cytotoxic versus cytoprotective effects. Chem. Res. Toxicol. 30, 13–37. 10.1021/acs.chemrestox.6b00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager J. D. (2015) Mechanisms of estrogen carcinogenesis: The role of E2/E1-quinone metabolites suggests new approaches to preventive intervention-A review. Steroids 99, 56–60. 10.1016/j.steroids.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap T. L.; Wang S.; Simmler C.; Chen S. N.; Pauli G. F.; Dietz B. M.; Bolton J. L. (2015) Differential effects of glycyrrhiza species on genotoxic estrogen metabolism: licochalcone A downregulates P450 1B1, whereas isoliquiritigenin stimulates it. Chem. Res. Toxicol. 28, 1584–1594. 10.1021/acs.chemrestox.5b00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reding K. W.; Zahid M.; Cavalieri E.; Rogan E. G.; Raccor B. S.; Atkinson C.; Yong M.; Newton K. M.; Lampe J. W. (2014) Associations between dietary intake of fruits and vegetables in relation to urinary estrogen DNA adduct ratio. Open J. Prev. Med. 4, 429. 10.4236/ojpm.2014.46050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Breemen R. B. (2015) Development of safe and effective botanical dietary supplements. J. Med. Chem. 58, 8360–8372. 10.1021/acs.jmedchem.5b00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson J. E.; Chlebowski R. T.; Stefanick M. L.; Aragaki A. K.; Rossouw J. E.; Prentice R. L.; Anderson G.; Howard B. V.; Thomson C. A.; LaCroix A. Z.; Wactawski-Wende J.; Jackson R. D.; Limacher M.; Margolis K. L.; Wassertheil-Smoller S.; Beresford S. A.; Cauley J. A.; Eaton C. B.; Gass M.; Hsia J.; Johnson K. C.; Kooperberg C.; Kuller L. H.; Lewis C. E.; Liu S.; Martin L. W.; Ockene J. K.; O’Sullivan M. J.; Powell L. H.; Simon M. S.; Van Horn L.; Vitolins M. Z.; Wallace R. B. (2013) Menopausal Hormone Therapy and Health Outcomes During the Intervention and Extended Poststopping Phases of the Women’s Health Initiative Randomized Trials. JAMA 310, 1353–1368. 10.1001/jama.2013.278040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton J. L. (2016) Chem. Res. Toxicol. 29, 1583–1590. 10.1021/acs.chemrestox.6b00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denison M. S.; Nagy S. R. (2003) Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu. Rev. Pharmacol. Toxicol. 43, 309–334. 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- Cavalieri E. L.; Rogan E. G. (2016) Depurinating estrogen-DNA adducts, generators of cancer initiation: their minimization leads to cancer prevention. Clin. Transl. Med. 5, 12. 10.1186/s40169-016-0088-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.-A.; Na H.-K.; Surh Y.-J. (2012) Resveratrol suppresses 4-hydroxyestradiol-induced transformation of human breast epithelial cells by blocking IκB kinaseβ-NF-κB signalling. Free Radical Res. 46, 1051–1057. 10.3109/10715762.2012.671940. [DOI] [PubMed] [Google Scholar]
- Park S. A.; Lee M. H.; Na H. K.; Surh Y. J. (2017) 4-Hydroxyestradiol induces mammary epithelial cell transformation through Nrf2-mediated heme oxygenase-1 overexpression. Oncotarget 8, 164–178. 10.18632/oncotarget.10516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemachandra L.; Madhubhani P.; Chandrasena R.; Esala P.; Chen S. N.; Main M.; Lankin D. C.; Scism R. A.; Dietz B. M.; Pauli G. F.; Thatcher G. R.; Bolton J. L. (2012) Hops (Humulus lupulus) inhibits oxidative estrogen metabolism and estrogen-induced malignant transformation in human mammary epithelial cells (MCF-10A). Cancer Prev. Res. 5, 73–81. 10.1158/1940-6207.CAPR-11-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager J. D.; Davidson N. E. (2006) Estrogen carcinogenesis in breast cancer. N. Engl. J. Med. 354, 270–282. 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- Bolton J. L.; Thatcher G. R. (2008) Potential mechanisms of estrogen quinone carcinogenesis. Chem. Res. Toxicol. 21, 93–101. 10.1021/tx700191p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri E. L., and Rogan E. G. (2016) Inhibition of depurinating estrogen-DNA adduct formation in the prevention of breast and other cancers, in Trends in Breast Cancer Prevention (Russo J., Ed.) pp 113–145, Springer International Publishing. [Google Scholar]
- Gupta M.; McDougal A.; Safe S. (1998) Estrogenic and antiestrogenic activities of 16α-and 2-hydroxy metabolites of 17β-estradiol in MCF-7 and T47D human breast cancer cells. J. Steroid Biochem. Mol. Biol. 67, 413–419. 10.1016/S0960-0760(98)00135-6. [DOI] [PubMed] [Google Scholar]
- Mueck A.; Seeger H. (2010) 2-Methoxyestradiol—biology and mechanism of action. Steroids 75, 625–631. 10.1016/j.steroids.2010.02.016. [DOI] [PubMed] [Google Scholar]
- Dong J.; Zhang Q.; Cui Q.; Huang G.; Pan X.; Li S. (2016) Flavonoids and naphthoflavonoids: wider roles in the modulation of cytochrome P450 Family 1 enzymes. ChemMedChem 11, 2102–2118. 10.1002/cmdc.201600316. [DOI] [PubMed] [Google Scholar]
- Snelten C. S.; Dietz B.; Bolton J. L. (2012) Modulation of estrogen chemical carcinogenesis by botanical supplements used for postmenopausal women’s health. Drug Discovery Today: Dis. Mech. 9, e47–e54. 10.1016/j.ddmec.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong P.; Madak-Erdogan Z.; Flaws J. A.; Shapiro D. J.; Katzenellenbogen J. A.; Katzenellenbogen B. S. (2016) Estrogen receptor-α and aryl hydrocarbon receptor involvement in the actions of botanical estrogens in target cells. Mol. Cell. Endocrinol. 437, 190–200. 10.1016/j.mce.2016.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodziejczyk-Czepas J. (2012) Trifolium species-derived substances and extracts-biological activity and prospects for medicinal applications. J. Ethnopharmacol. 143, 14–23. 10.1016/j.jep.2012.06.048. [DOI] [PubMed] [Google Scholar]
- Geller S. E.; Shulman L. P.; van Breemen R. B.; Banuvar S.; Zhou Y.; Epstein G.; Hedayat S.; Nikolic D.; Krause E. C.; Piersen C. E.; Bolton J. L.; Pauli G. F.; Farnsworth N. R. (2009) Safety and efficacy of black cohosh and red clover for the management of vasomotor symptoms: a randomized controlled trial. Menopause 16, 1156–1166. 10.1097/gme.0b013e3181ace49b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco O. H.; Chowdhury R.; Troup J.; Voortman T.; Kunutsor S.; Kavousi M.; Oliver-Williams C.; Muka T. (2016) Use of plant-based therapies and menopausal symptoms A systematic review and meta-analysis. J. Am. Med. Assoc. 315, 2554–2563. 10.1001/jama.2016.8012. [DOI] [PubMed] [Google Scholar]
- Overk C. R.; Yao P.; Chadwick L. R.; Nikolic D.; Sun Y.; Cuendet M. A.; Deng Y.; Hedayat A. S.; Pauli G. F.; Farnsworth N. R.; van Breemen R. B.; Bolton J. L. (2005) Comparison of the in vitro estrogenic activities of compounds from hops (Humulus lupulus) and red clover (Trifolium pratense). J. Agric. Food Chem. 53, 6246–6253. 10.1021/jf050448p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S. Y.; Ha T. Y.; Ahn J. Y.; Kim S. R.; Kang K. S.; Hwang I. K.; Kim S. (2008) Estrogenic activities of isoflavones and flavones and their structure-activity relationships. Planta Med. 74, 25–32. 10.1055/s-2007-993760. [DOI] [PubMed] [Google Scholar]
- Peterson T. G.; Coward L.; Kirk M.; Falany C. N.; Barnes S. (1996) The role of metabolism in mammary epithelial cell growth inhibition by the isoflavones genistein and biochanin A. Carcinogenesis 17, 1861–1869. 10.1093/carcin/17.9.1861. [DOI] [PubMed] [Google Scholar]
- Bialesova L.; Novotna A.; Macejova D.; Brtko J.; Dvorak Z. (2015) Agonistic effect of selected isoflavones on arylhydrocarbon receptor in a novel AZ-AhR transgenic gene reporter human cell line. Gen. Physiol. Biophys. 34, 331–334. 10.4149/gpb_2015008. [DOI] [PubMed] [Google Scholar]
- Chan H. Y.; Wang H.; Leung L. K. (2003) The red clover (Trifolium pratense) isoflavone biochanin A modulates the biotransformation pathways of 7,12-dimethylbenz[a]anthracene. Br. J. Nutr. 90, 87–92. 10.1079/BJN2003868. [DOI] [PubMed] [Google Scholar]
- Medjakovic S.; Jungbauer A. (2008) Red clover isoflavones biochanin A and formononetin are potent ligands of the human aryl hydrocarbon receptor. J. Steroid Biochem. Mol. Biol. 108, 171–177. 10.1016/j.jsbmb.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Han E. H.; Kim J. Y.; Jeong H. G. (2006) Effect of biochanin A on the aryl hydrocarbon receptor and cytochrome P450 1A1 in MCF-7 human breast carcinoma cells. Arch. Pharmacal Res. 29, 570–576. 10.1007/BF02969267. [DOI] [PubMed] [Google Scholar]
- Han E.-H.; Jeong T.-C.; Jeong H.-G. (2007) Effects of formononetin on the aryl hydrocarbon receptor and 7,12-dimethylbenz [a] anthracene-induced cytochrome P450 1A1 in MCF-7 human breast carcinoma cells. Toxicol. Res. 23, 135–142. 10.5487/TR.2007.23.2.135. [DOI] [Google Scholar]
- Booth N. L; Overk C. R.; Yao P.; Burdette J. E; Nikolic D.; Chen S.-N.; Bolton J. L; van Breemen R. B; Pauli G. F; Farnsworth N. R (2006) The chemical and biologic profile of a red clover (Trifolium pratense L.) phase II clinical extract. J. Altern. Complement. Med. 12, 133–139. 10.1089/acm.2006.12.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S. Y.; Wolf D. M.; Yingling J. M.; Chang C.; Jordan V. C. (1992) An estrogen-receptor positive MCF-7 Clone that is resistant to antiestrogens and estradiol. Mol. Cell. Endocrinol. 90, 77–86. 10.1016/0303-7207(92)90104-E. [DOI] [PubMed] [Google Scholar]
- Marques M.; Laflamme L.; Gaudreau L. (2013) Estrogen receptor alpha can selectively repress dioxin receptor-mediated gene expression by targeting DNA methylation. Nucleic Acids Res. 41, 8094–8106. 10.1093/nar/gkt595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz B. M.; Hajirahimkhan A.; Dunlap T. L.; Bolton J. L. (2016) Botanicals and their bioactive phytochemicals for women’s health. Pharmacol. Rev. 68, 1026–1073. 10.1124/pr.115.010843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spink D. C.; Spink B. C.; Cao J. Q.; DePasquale J. A.; Pentecost B. T.; Fasco M. J.; Li Y.; R S. T. (1998) Differential expression of CYP1A1 and CYP1B1 in human breast epithelial cells and breast tumor cells. Carcinogenesis 19, 291–298. 10.1093/carcin/19.2.291. [DOI] [PubMed] [Google Scholar]
- Ricketts D.; Turnbull L.; Ryall G.; Bakhshi R.; Rawson N.; Gazet J.; Nolan C.; Coombes R. (1991) Estrogen and progesterone receptors in the normal female breast. Cancer Res. 51, 1817–1822. [PubMed] [Google Scholar]
- Burdette J. E.; Liu J.; Lantvit D.; Lim E.; Booth N.; Bhat K. P.; Hedayat S.; Van Breemen R. B.; Constantinou A. I.; Pezzuto J. M. (2002) Trifolium pratense (red clover) exhibits estrogenic effects in vivo in ovariectomized Sprague-Dawley rats. J. Nutr. 132, 27–30. [DOI] [PubMed] [Google Scholar]
- Powles T. J.; Howell A.; Evans D. G.; McCloskey E. V.; Ashley S.; Greenhalgh R.; Affen J.; Flook L. A.; Tidy A. (2008) Red clover isoflavones are safe and well tolerated in women with a family history of breast cancer. Menopause Int. 14, 6–12. 10.1258/mi.2007.007033. [DOI] [PubMed] [Google Scholar]
- Atkinson C.; Warren R. M.; Sala E.; Dowsett M.; Dunning A. M.; Healey C. S.; Runswick S.; Day N. E.; Bingham S. A. (2004) Red clover-derived isoflavones and mammographic breast density: a double-blind, randomized, placebo-controlled trial [ISRCTN42940165]. Breast Cancer Res. 6, 170–179. 10.1186/bcr773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher B. A.; Cotterchio M.; Anderson L. N.; Kreiger N.; Kirsh V. A.; Thompson L. U. (2013) Use of isoflavone supplements is associated with reduced postmenopausal breast cancer risk. Int. J. Cancer 132, 1439–1450. 10.1002/ijc.27769. [DOI] [PubMed] [Google Scholar]
- Amer D. A.; Kretzschmar G.; Müller N.; Stanke N.; Lindemann D.; Vollmer G. (2010) Activation of transgenic estrogen receptor-beta by selected phytoestrogens in a stably transduced rat serotonergic cell line. J. Steroid Biochem. Mol. Biol. 120, 208–217. 10.1016/j.jsbmb.2010.04.018. [DOI] [PubMed] [Google Scholar]
- Leung H. Y.; Yung L. H.; Poon C. H.; Shi G.; Lu A.-L.; Leung L. K. (2009) Genistein protects against polycyclic aromatic hydrocarbon-induced oxidative DNA damage in non-cancerous breast cells MCF-10A. Br. J. Nutr. 101, 257–262. 10.1017/S0007114508998457. [DOI] [PubMed] [Google Scholar]
- de Lemos M. L. (2001) Effects of soy phytoestrogens genistein and daidzein on breast cancer growth. Ann. Pharmacother. 35, 1118–1121. 10.1345/aph.10257. [DOI] [PubMed] [Google Scholar]
- Hsieh C.-Y.; Santell R. C.; Haslam S. Z.; Helferich W. G. (1998) Estrogenic effects of genistein on the growth of estrogen receptor-positive human breast cancer (MCF-7) cells in vitro and in vivo. Cancer Res. 58, 3833–3838. [PubMed] [Google Scholar]
- Lehmann L.; Jiang L.; Wagner J. (2008) Soy isoflavones decrease the catechol-O-methyltransferase-mediated inactivation of 4-hydroxyestradiol in cultured MCF-7 cells. Carcinogenesis 29, 363–370. 10.1093/carcin/bgm235. [DOI] [PubMed] [Google Scholar]
- Zhang S.; Qin C.; Safe S. H. (2003) Flavonoids as aryl hydrocarbon receptor agonists/antagonists: effects of structure and cell context. Environ. Health Perspect. 111, 1877. 10.1289/ehp.6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J.; Jiang L.; Lehmann L. (2008) Phytoestrogens modulate the expression of 17a-estradiol metabolizing enzymes in cultured MCF-7 cells. Advances in experimental medicine and biology 617, 625–632. 10.1007/978-0-387-69080-3_65. [DOI] [PubMed] [Google Scholar]
- Safe S.; Wormke M.; Samudio I. (2000) Mechanisms of inhibitory aryl hydrocarbon receptor-estrogen receptor crosstalk in human breast cancer cells. J. Mammary Gland Biol. Neoplasia 5, 295–306. 10.1023/A:1009550912337. [DOI] [PubMed] [Google Scholar]
- Spink D. C.; Katz B. H.; Hussain M. M.; Pentecost B. T.; Cao Z.; Spink B. C. (2003) Estrogen regulates Ah responsiveness in MCF-7 breast cancer cells. Carcinogenesis 24, 1941–1950. 10.1093/carcin/bgg162. [DOI] [PubMed] [Google Scholar]
- Tsuchiya Y.; Nakajima M.; Kyo S.; Kanaya T.; Inoue M.; Yokoi T. (2004) Human CYP1B1 is regulated by estradiol via estrogen receptor. Cancer Res. 64, 3119–3125. 10.1158/0008-5472.CAN-04-0166. [DOI] [PubMed] [Google Scholar]
- Beischlag T. V.; Perdew G. H. (2005) ER alpha-AHR-ARNT protein-protein interactions mediate estradiol-dependent transrepression of dioxin-inducible gene transcription. J. Biol. Chem. 280, 21607–21611. 10.1074/jbc.C500090200. [DOI] [PubMed] [Google Scholar]
- Osborne C. K.; Hobbs K.; Trent J. M. (1987) Biological differences among MCF-7 human breast cancer cell lines from different laboratories. Breast Cancer Res. Treat. 9, 111–121. 10.1007/BF01807363. [DOI] [PubMed] [Google Scholar]
- Nugoli M.; Chuchana P.; Vendrell J.; Orsetti B.; Ursule L.; Nguyen C.; Birnbaum D.; Douzery E. J.; Cohen P.; Theillet C. (2003) Genetic variability in MCF-7 sublines: evidence of rapid genomic and RNA expression profile modifications. BMC Cancer 3, 13. 10.1186/1471-2407-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahia H.; Ashman J.; Cawkwell L.; Lind M.; Monson J.; Drew P.; Greenman J. (2002) Karyotypic variation between independently cultured strains of the cell line MCF-7 identified by multicolour fluorescence in situ hybridization. Int. J. Oncol. 20, 489–494. 10.3892/ijo.20.3.489. [DOI] [PubMed] [Google Scholar]
- Piersen C.; Booth N.; Sun Y.; Liang W.; Burdette J.; Breemen R. v.; Geller S.; Gu C.; Banuvar S.; Shulman L.; Bolton J.; Farnsworth N. (2004) Chemical and biological characterization and clinical evaluation of botanical dietary supplements: a phase I red clover extract as a model. Curr. Med. Chem. 11, 1361–1374. 10.2174/0929867043365134. [DOI] [PubMed] [Google Scholar]
- Peterson T. G.; Ji G.-P.; Kirk M.; Coward L.; Falany C. N.; Barnes S. (1998) Metabolism of the isoflavones genistein and biochanin A in human breast cancer cell lines. Am. J. Clin. Nutr. 68, 1505–1511. [DOI] [PubMed] [Google Scholar]
- Kligman L.; Younus J. (2010) Management of hot flashes in women with breast cancer. Curr. Oncol. 17, 81. 10.3747/co.v17i1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.