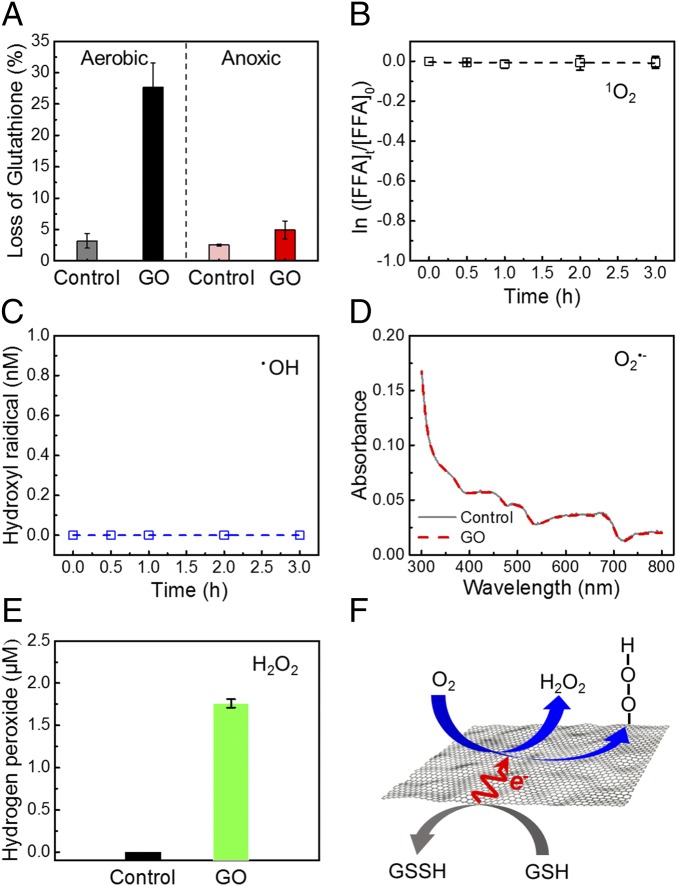
Fig. 6.
Major role of electron-transfer oxidation in chemical toxicity of GO nanosheets. (A) In vitro glutathione oxidation by GO nanosheets in suspension. Glutathione (0.4 mM) was exposed to 0 (control) or 200 μg⋅mL−1 GO in bicarbonate buffer (50 mM, pH 8.6) at room temperature for 3 h. (Left) For the aerobic condition, the solution was prepared without any treatment and the dissolved oxygen was ∼8.7 mg⋅L−1. (Right) For the anoxic condition, the solution was purged with nitrogen gas for 30 min to decrease the dissolved oxygen to ∼0.4 mg⋅L−1. (B–E) ROS generation in the 200 μg⋅mL−1 GO solution detected with ROS probes. The experiments were conducted in the dark for 3 h at room temperature. (B) Generation of 1O2 is indicated by decay of furfuryl alcohol (FFA; initial concentration of 50 μM). (C) Cumulative •OH generation over time is indicated by the formation of hydroxyterephthalate. (D) Generation of O2•− indicated by the reduction of 2,3-Bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (initial concentration of 100 μM). (E) H2O2 concentration measured by the Amplex Red assay. (F) Schematic illustrating electron-transfer oxidation of a model intracellular compound, glutathione (GSH), mediated by a GO nanosheet. Each glutathione molecule donates one electron to form its corresponding oxidized dimer, glutathione disulfide (GSSH). The GO nanosheet works as an electron shuttle to transfer electrons to dissolved oxygen, which subsequently forms H2O2 or surface-bound peroxide intermediates at edge or defect sites of GO nanosheets.