Significance
Growth of NIH 3T3 cells in an optimized medium produced transformed foci after reaching confluence in the absence of treatment with carcinogens or transfection with tumor DNA. Dynamic experiments showed the first response of the crowded cells was gradual population-wide increases in saturation density followed by transformed foci. The latter produced malignant tumors when injected into mice. The field of cells around the foci became increasingly susceptible to transformation. Analysis of the data revealed that transformation was driven by epigenetic selection of cell populations that grew well at high densities rather than genetic instability. That conclusion is reinforced by two other methods of selection, one at low cell density in low serum concentration and the other by suspension in agar.
Keywords: selection, saturation density, cellular microenvironment, epigenetics
Abstract
NIH 3T3 cells grown in conventional Dulbecco’s modification of Eagle’s basal medium (DME) produce no transformed foci when grown to confluence in 10% calf serum (CS). A few cultures were transformed by ras oncogenes when transfected with DNA from neoplastic cells, but they failed to do so in 80 to 90% of the transfections. However, when they were grown in a medium [molecular, cellular, and developmental biology 402 (MCDB 402)] optimized for their clonal growth in minimal serum, they produced transformed foci without transfection in 10% CS, but not in 2% CS. The first response to growth in MCDB 402 in 2% CS in successive rounds of contact inhibition was uniform increases in saturation density of the population. This was followed by the appearance of transformed foci. A systematic study was made of the dynamics of neoplastic progression in various concentrations of CS in a single round of confluence at 2 and 3 wk, followed by three sequential rounds of confluence in 2% CS for 2 wk. There was a linear relationship between CS concentration and saturation density in the first-round cultures and continuing differences in subsequent cultures. The hyperplastic field of normal-looking cells surrounding transformed foci became increasingly permissive for transformation with serial culture. The dynamics show that epigenetic selection is the major driving force of neoplastic development. Cells from dense foci produced malignant fibrosarcomas in mice, thereby exhibiting a positive relationship between transformation in culture and the development of tumors.
NIH 3T3 cells are a clonal line of mouse embryo fibroblasts derived from randomly bred Swiss mice (1). The cells were selected for their high cloning efficiencies and low saturation density. They were thought to be susceptible to the oncogenic activity of nuclear DNA transfected from spontaneous human tumors and from cells transformed in vivo or in vitro by a variety of carcinogenic treatments (2). Transfection was carried out by the calcium phosphate coprecipitation technique of Graham and Van der Eb (3). Transformation was expressed in the transfected NIH 3T3 cells by production of foci, which had the capacity to produce sarcomas when injected into immunodeficient mice. The causal agents in a high proportion of cases were subtypes of the ras oncogene, but there was a failure to observe transforming activity in 80 to 90% of spontaneous human tumors. In addition, the numbers of foci were generally low and often erratic (4).
Despite their limited susceptibility to transformation after transfection by DNA from neoplastic tissue, the NIH 3T3 cells played a significant role in the development of the molecular genetics of cellular oncogenes (2). However, nontransfected, control monolayers of NIH 3T3 cells or unrelated sources of transfected DNA occasionally induced transformed foci, which were generally interpreted as spontaneous transformation in NIH 3T3 cells (4–6).
Little attention was paid to these seemingly extraneous foci, but later work provided explanation for them as will be seen below. The culture medium for the NIH 3T3 cells in the above experiments (4–8) and many other experiments along the same line (2) was the empirically applied Dulbecco’s modification of Eagle’s basal medium (DME) or minor modifications supplemented with 10% serum.
However, a medium [molecular, cellular, and developmental biology 402 (MCDB 402)] was developed aimed at more definition and reduction of serum for optimal clonal growth of Swiss 3T3 cells, the precursor of the NIH 3T3 cells (9). The preparation of MCDB 402 was fastidiously adjusted for concentrations of all of the constituents of DME to optimum values for clonal growth of the cells and added such components as “nonessential” amino acids and some vitamins and other organic compounds, as well as trace elements. The doubling time for clonal growth of NIH 3T3 cells in MCDB 402 serum was 12 h (10) while it was 18 to 22 h for growth of the closely related Balb 3T3 cells in DME (11).
All 13 of the amino acids considered “essential” for cells in culture were included in both DME and MCDB 402, but the latter contained all of the remaining amino acids, of which asparagine was missing in DME, but found essential for clonal growth in MCDB 402 in very low concentrations of dialyzed and lyophilized FBS protein (FBSP). An unusual aspect of MCDB 402 was a very high concentration of histidine and a very low concentration of tryptophan (molar ratio 200:1). MCDB 402 contains a total of nine vitamins, six of which are required for clonal growth of the Swiss 3T3 cells in minimal protein, and one of three of which is not present in DME. Other organic compounds, such as linoleic acid and putrescine, are not present in DME but were found beneficial in MCDB 402. Nine trace elements are included in MCDB 402, but there are none in DME, yet iron is essential for growth, and copper, manganese, and zinc are beneficial. Multiplication is reduced when any of them is omitted, and no colony formation occurs when they are all omitted simultaneously. Positively charged polylysine was used to coat the culture vessels to substitute for serum protein in very low concentrations of the latter (12). MCDB 402 was approximately five times more effective than DME in promoting the colony-forming efficiency and colony size of Swiss 3T3 cells in very low concentrations of FBSP.
Transformed Focus Formation at High Cell Densities of NIH 3T3 Cells in MCDB 402 Without Transfection by Oncogenes
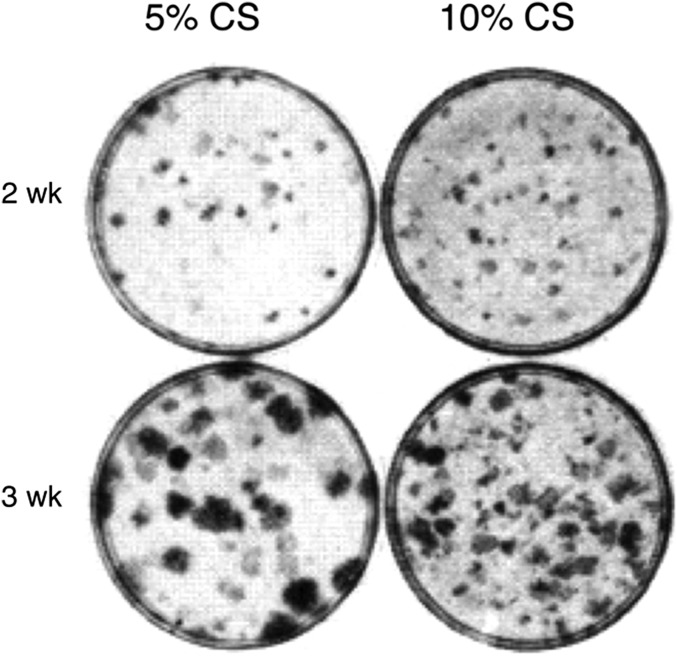
The NIH 3T3 cells were kept in MCDB 402 without transformation by weekly passage at very low cell number: 104 per 60-mm culture dish in 10% calf serum (CS). They were subcultured at 105 cells in 10%, 5%, and 2% serum (13). They reached confluence in 3 to 4 d and were fixed and stained at 2 and 3 wk (Fig. 1). The assay for transformation was the appearance of countable foci made up of cells that continued to multiply on a background of monolayered, contact-inhibited, nontransformed cell culture. The cultures were stained with a dye that accentuates the density and countability of the transformed foci. There were many very small transformed foci of different densities in 5% CS at 2 wk and more small and denser foci in 10% CS. At 3 wk, the foci in 5% CS had become larger and dense, but smaller, less dense foci were evident in the background. There were many more visible foci in 10% CS than in 5% CS although they were smaller and less dense than those in 5% CS, very likely because their very large number in 10% CS had depleted the medium. There were no foci in the cultures assayed in 2% CS.
Fig. 1.
Transformed focus formation in early passage NIH 3T3 cells in MCDB 402. Cells (105) were seeded per 60-mm dishes in the indicated concentrations of CS and time of incubation (13). The number of foci remained constant when 104 cells were passaged weekly, and 105 cells were assayed in 5% and 10% CS at each passage. They were then incubated for 2 and 3 wk before staining.
However, the question arose whether the capacity to produce foci in 2% CS increased when the cells in 10% CS were multiplying or after they reached their saturation density. The results of the appropriate experiment showed that the capacity of the cells in 10% CS for focus formation in 2% CS increased only several days after the former had reached their saturation density (13), indicating that the foci and preceding events were predominantly derived by selection rather than by spontaneous mutations in multiplying cells.
The Dynamics of Progression Through Pre- and Postneoplastic Development
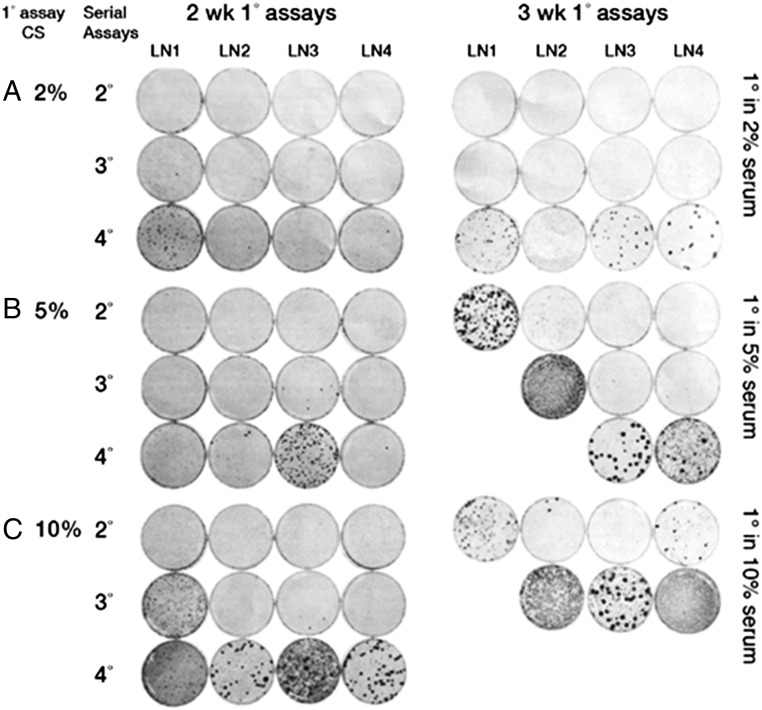
The study of progressive dynamics proceeded as follows (10, 14). The saturation density of cultures started in the 1° (primary) assay for 2 wk had a linear relation with CS concentration (Table 1). All of the lineages derived from each of the original CS concentrations had increased in the 2° assay, which was conducted under constant conditions (2% serum for 2 wk) in nonlinear proportion to the CS concentrations of the 1° assay (Table 2). Those started from 2% CS for 2 wk showed uniform increases in saturation densities in each of the subsequent serial assays whereas those started from 5% CS for 2 wk showed uniform increases only through the 3° assay, and those from the original 10% CS for 2 wk only through the 2° assay (Table 2). The 4° assay that arose from the original 5% CS for 2 wk exhibited foci in one of the four lineages (Fig. 2). Those that arose from the original 10% CS for 2 wk had one lineage with foci in the 3° assay. In the 4° assay of this category, three lineages exhibited many foci, and the fourth displayed confluent transformation.
Table 1.
Saturation densities of the first round of selection (1° assay) in various concentrations of CS and times of incubation (14)
| CS, % | Cell count, x10−5 | |
| 2 wk | 3 wk | |
| 2 | 4.3 ± 0.1 | 4.7 ± 0.1 |
| 5 | 9.6 ± 0.5 | 11.3 ± 0.4 |
| 10 | 21.4 ± 0.4 | 23.0 ± 0.9 |
Table 2.
Transformation is accelerated by preincubation with higher concentrations of serum
| Assay | CS % | Saturation density, cells (10−5) per culture | |||||||||
| 2-wk 1° assay | 3-wk 1° assay | ||||||||||
| LN1 | LN2 | LN3 | LN4 | Average | LN1 | LN2 | LN3 | LN4 | Average | ||
| 2% CS 1° | |||||||||||
| 1° | 2 | 4.3 | 4 | 4.3 | 4.5 | 4.28 ± 0.12 | 4.6 | 4.7 | 4.8 | 4.6 | 4.68 ± 0.06 |
| 2° | 2 | 4.8 | 4.6 | 4.1 | 4.9 | 4.6 ± 0.21 | 5.5 | 5.5 | 5.1 | 5.2 | 5.32 ± 0.12 |
| 3° | 2 | 7.7 | 7.6 | 6.6 | 7.1 | 7.25 ± 0.29 | 9.4 | 9 | 8.2 | 8.4 | 8.75 ± 0.32 |
| 4° | 2 | 9.7 | 9.8 | 10.1 | 5.3 | 8.73 ± 1.32 | (12.6) | 9.3 | (12.4) | 13.9 | |
| 5% CS 1° | |||||||||||
| 1° | 5 | 9.6 | 8.4 | 10.1 | 10.2 | 9.57 ± 0.48 | 11.2 | 12.1 | 10.7 | 11.2 | 11.3 ± 0.33 |
| 2° | 2 | 5.8 | 5 | 6.2 | 6 | 5.75 ± 0.31 | (56.5) | 7.4 | 7.8 | 6.6 | 7.27 ± 0.35 |
| 3° | 2 | 7.7 | 7.7 | 8.9 | 7.5 | 7.95 ± 0.37 | ND | (76.5) | 9.8 | 10.8 | 10.30 ± 0.71 |
| 4° | 2 | 9.1 | 9.9 | (15.0) | 9.1 | 9.37 ± 0.27 | ND | ND | (16.3) | (18.0) | |
| 10% CS 1° | |||||||||||
| 1° | 10 | 22.1 | 21.8 | 21.2 | 20.3 | 21.32 ± 0.46 | 22.4 | 22.8 | 18.7 | 23.7 | 21.90 ± 1.27 |
| 2° | 2 | 7.5 | 6.6 | 6.6 | 7.3 | 7.0 ± 0.27 | 30 | 9.8 | 9.4 | (11.9) | 9.6 ± 0.28 |
| 3° | 2 | (19.2) | 9.2 | 7.6 | 8.7 | 8.5 ± 0.47 | ND | (70.4) | (69.0) | (55.1) | |
| 4° | 2 | (56.4) | (22.9) | (54.5) | (26.3) | ND | ND | ND | ND | ||
Saturation densities of cells under varying concentrations of CS and time in a 1° assay at confluence for 2 and 3 wk, switched to constant 2% CS and 2 wk in 2°, 3° and 4° assays. Four lineages in each category were grown at high density in a 1° assay in combinations of 2%, 5%, and 10% CS for 2 and 3 wk (i.e., six categories). They were then passaged in 2% CS for 2 wk in serial 2°, 3°, and 4° assays. Saturation densities were recorded at each passage, and averages were determined. The averages excluded cultures that had dense foci since they (denoted by parentheses) raised the saturation density significantly above those with no foci. ND signifies a culture that was “not done” because the previous assay had more than fivefold greater saturation density than the average of the 1° assay in 2% CS for 2 wk.
Fig. 2.
Sister cultures in 2°, 3°, and 4° assays equivalent to those in Table 2 but fixed and stained for foci.
All of the series that spent 3 wk in the 1° assays showed higher saturation densities than their corresponding serum concentration from 2 wk in the 1° assay. The shift to transformed foci occurred earlier in the cultures derived from the 3-wk 1° assay with stronger foci and some that had advanced to confluent transformation. In those lineages where dense foci occurred, there was a sharp increase in saturation density (Fig. 2 and Table 2). If those lineages with sharp increases in saturation density are excluded from calculation of the average saturation densities, there was a continuous increase in that parameter that indicates selection of a large number of heritable changes that have population-wide increases in saturation density, indicating the accumulation of large numbers of heritable changes of low fitness advantage. In contrast, the onset of transformed foci had a stochastic basis of high fitness advantage consistent with their origin from single cells (Fig. 2).
Dynamics of Contact Inhibition in the Circumfocal Area Made Up of Nontransformed Cells
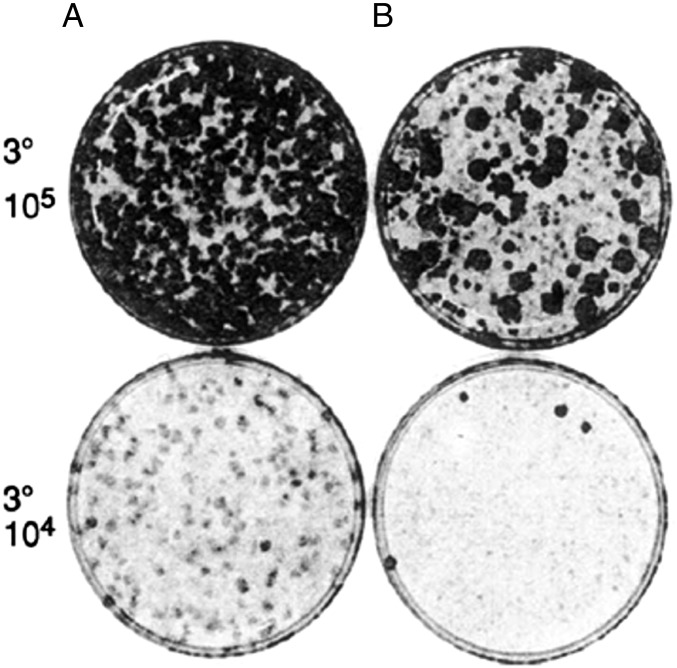
It is widely recognized that the loss of contact inhibition is an essential feature of cellular transformation (11). As shown above, there is an increase in saturation density of the entire population of cells in successive rounds of selection before transformed foci appear, suggesting that all of the cells undergo progressive degrees of the loss of contact inhibition. That would indicate that a field of increasing permissiveness precedes transformation. That was demonstrated in round 3° of selection where the cultures started in the first round in 10% CS for 3 wk formed hundreds of dense foci in seeding 105 of the cells in lineage A, and 40 large, dense foci in lineage B (15) (Fig. 3). A 10-fold dilution of the same cells was seeded together with 105 cells that had never undergone selection to form a confluent background for the transformed cells (Fig. 3). There were no dense foci in the diluted cells of lineage A, and four small, dense foci of lineage B, with the complete disappearance of small, light foci of the 105 undiluted cells of lineage 3. The results show that the background cells that had undergone three rounds of selection were more permissive for the growth of the transformed cells than were the original nonselected background cells.
Fig. 3.
Increasing permissiveness of transformed focus formation by serial selection of their cellular microenvironment (15). Two lineages, A and B, underwent a 1° assay in 10% CS for 3 wk, followed by 2° and 3° assays in 2% CS for 3 wk. The 3° assay at 105 cells is shown, as is that of 104 cells combined with 105 cells that had not undergone a selective assay to serve as a background for focus formation. Reproduced from ref. 15.
Relation Between Transformed Foci and Their Tumorigenicity in Mice
NIH 3T3 cells undergo transformation when passaged frequently at low density in 2% CS (16). Transformed cells were isolated from a dense focus and passaged frequently in either 2% or 10% CS (17). Those passaged in 2% CS retained their capacity for transformed focus formation and tumor production at 6 and 34 passages (Table 3). When passaged six times in 10% CS, they lost half their capacity to induce focus formation although their capacity for tumor production remained high. However, their capacity for focus formation was totally lost at 34 passages in 10% CS while the capacity for tumor production was lost in three of four (3/4) mice. The latency of the 1/4 positive that produced a tumor was four times longer than the average latencies of the four groups that had 4/4 positives. The same was true for the 1/4 positive of the control, indicating that those two tumors with latent periods of 97 d became transformed after injection of the cells. The results suggest a strong relationship between dense focus formation and tumorigenicity. Cells from the explant of a tumor that had been initiated by injection of cells from a dense focus had a large number of dense foci and tumors with the extremely short latent period of 5 d. Comparison with the much longer, average latent period of 25 d of cells directly injected from dense foci indicates that the latter had to undergo an adaptation period for tumor formation. The results from light foci were erratic in focus formation and tumor development (17).
Table 3.
Relation between dense, transformed foci and their tumorigenicity in mice
| Passages | Foci per 100 cells | Tumors | Latency, d | |
| 2% CS | 10% CS | |||
| 6 | 0 | 65, 59 | 4/4 | 25 |
| 34 | 0 | 48, 50 | 4/4 | 33 |
| 0 | 6 | 24, 26 | 4/4 | 25 |
| 0 | 34 | 0, 0 | 1/4 | 97* |
| 1 | 0 | 39, 34 | 4/4 | 18 |
| Explant | ||||
| 1 | 0 | 55, 53 | 4/4 | 5 |
| Control | ||||
| 0 | 86 | 0, 0 | 1/4 | 97† |
Cells were isolated from a dense, transformed focus that appeared during passage of cells 3 times per wk at low density in 2% CS. They were passaged at the same frequency in either 2% or 10% CS, and 100 cells of each were assayed for focus formation at passages 6 and 34 and mixed with 105 nontransformed cells. Tumorigenicity was determined by s.c. injection of 5 × 105 cells into athymic mice, which were checked weekly for tumor formation. Cells were also isolated from a lightly stained, partly transformed focus but gave erratic results in both transformed foci and tumors. An interesting sidelight is that the explant of a tumor that had been initiated by cells from a dense focus and passaged once in 2% CS yielded cells that produced tumors with the extremely short latent period of 5 d.
Only one of four injected animals in the 10% CS group, but the latent period was four times longer than the average of the groups that had 4/4 positives.
The control group consisting of nontransformed cells passaged in 10% CS had 1/4 positive, with a prolonged latent period the same as that of the * group, suggesting that both were initiated after injection.
Discussion
Since development of cancer is thought to be clonal, the results presented here illustrate the general importance of using medium that is optimized for clonal growth (MCDB 402) to reveal the full range of neoplastic progression by NIH 3T3 cells (9). The same medium was also optimized for growth of Balb 3T3 cells (18), with properties virtually identical to those of the NIH 3T3 cells (19). The Balb 3T3 cells were used to establish the heterogeneity of normal cells for growth on plastic (20) and of the capacity of growth in agar of transformed cells (21). That heterogeneity underlay the progressive selection of NIH 3T3 cells for transformation by serial rounds of contact inhibition in MCDB 402 (13). Such sequential progression did not appear in experiments involving DNA oncogene transfection of cells, which were growing in DME, which lacked many of the components necessary to exhibit this process. For comparative consistency of model systems, we used the NIH 3T3 cells in clonal medium MCDB 402 in our experiments presented here, which reexamine the dynamic aspects of neoplastic development (2).
DNA transfection of presumed oncogenic DNA into NIH 3T3 cells growing in DME failed to yield transformed foci using DNA from 80 to 90% of spontaneous human cancers (2). Despite this, the few successful transformations by the transfection of tumor DNA supported the common belief of that era (1958–2000) that cancer was caused by single oncogenes, which was initially based on results from transformation of chicken embryo cells by Rous sarcoma virus (RSV) (22). DNA from cells transformed by RSV was highly successful in transforming NIH 3T3 cells, which gives added importance to the general failure of the DNA from spontaneous human cancers. That means that one of the main supports for mutation as the cause of cancer is not valid. However, transformation by RSV infection proved an inappropriate model for spontaneous human cancers for several reasons. For example, ectodermal tumors on the chorioallantoic membrane of chicken embryos induced by RSV infection undergo massive necrosis within a few days after their formation (23). Fibroblasts in cell culture lose the capacity to multiply when the majority of them are infected by a high dose of RSV although they make transformed foci when surrounded by normal cells (24). FBS protein (FBSP) at conventional concentrations, or high concentrations of calf serum, prevent the morphological transformation of RSV-infected cells in culture (25) whereas the rate of spontaneous transformation is greatly enhanced by raising serum concentrations as shown here. Perhaps the most significant departure from the long-term, complex biology of cancer in humans (26) is that transformation by RSV occurs in a single step within a few days after RSV infection in contrast to the protracted preneoplastic phase of human cancer (27).
Failure of most transfections of tumor-associated DNA to produce transformation might be due to the need for increases in saturation density associated with increases in susceptibility to transformation. Those increases would not have occurred when only tumor DNA was transfected without the selective conditions that promote transformation. The dynamic aspects of neoplastic development were brought out by the scheme of varying the serum and concentration and time of the first round of selection, followed by repeated rounds under constant conditions. It revealed that the rate and extent of saturation density in rank order of the 1° assay was maintained under the constant condition of the subsequent serial assays, indicating that many of the increases in saturation density of the 1° assay resulted from mutations and/or stable epigenetic events.
In lieu of the failure of reductionist mechanisms, a functional biological approach to understanding the process of field cancerization was developing. Ironically, enough, it arose from use of the same NIH 3T3 cells, which had seemed to support the oncogene theory of tumor development. The main feature of that functional approach is consistent with the general insight of Werner Heisenberg, a founder of quantum mechanics: “Modern science has demonstrated that in the real world surrounding us, it is not the geometric (i.e., molecular?) forms but the dynamic laws governing movement (coming into being and passing away), which are permanent” (28). The dynamic aspects of neoplastic development were revealed in the experiment in which NIH 3T3 cells in MCDB 402 medium were grown to confluence under six different combinations. The preneoplastic increases in saturation density involved the entire cell population, suggesting uniform preneoplastic increments of very large numbers of mutational and/or epigenetic events of low fitness advantage distributed among all of the cells. That saturation slowly increased in the last assays derived from low serum concentration of the first assay. Tiny, barely visible foci arose in only one of four lineages of the last assay that originated from the 2-wk 1° assay, but many small, much denser foci arose in three of the four lineages after the final assay started from the 3-wk 1° assay. This extended delay of transformation could be interpreted as the cell culture equivalent of the decades-long, preneoplastic development of human cancer (26). The results conform to the finding in human colorectal cancer that more than 80% of the mutations in tumors originated in the preneoplastic state (27). The population-wide preneoplastic variations are consistent with the finding of 1,000 to 10,000 mutations in most human cancers, rising to as many as 100,000 in colon cancer and melanomas (29).
There is no evidence for a preneoplastic stage in transformed cells initiated by RSV infection. Therefore, it is not surprising that transfected DNA from such cells had a high incidence of transformed foci in NIH 3T3 cells (5). However, only a small fraction of the DNA from human tumors elicited transformation when transfected into NIH 3T3 cells (7). The low efficiency of transfection of DNA from human tumors into NIH 3T3 cells is consistent with the low fitness of thousands of preneoplastic mutations or of epigenetic processes, which underlie the development of human tumors (27, 29, 30).
By virtue of the finding that the Swiss mouse embryo cells can be promoted to neoplastic transformation by simple selection at high cell density, they might be considered “initiated” cells and not relevant to truly normal cells (31). However, freshly explanted, normal mouse embryo cells serially passaged at high cell density (i.e., in contact with each other) markedly increases their saturation density (32). Because they were randomly bred, their tumorigenic capacity could not at that time be tested in mice, but cells freshly explanted from the inbred Balb 3T3 line subjected to the same selective treatment with the same increases in saturation density were highly tumorigenic in mice (11). Increased saturation density was interpreted as the most reliable in vitro marker for tumorigenicity. The overall results are consistent with the evidence that hyperplasia is the first stage of tumor development, with ability to form tumors in the succeeding stages (33, 34).
The question arises what is the significance of the in vitro results for the more complex in vivo microenvironment. However, hyperplasia beyond normal tissue architecture is a prominent feature of field cancerization (33, 34). There are variations in growth factor activity of estrogens in women during pregnancy, which advances the growth of normal mammary epithelium (35). Transplanted mammary tumors of mice grow much more quickly in females than in males. However, implantation of stilbestrol pellets into males allows growth of injected mammary cancer cells at a rate equal to that seen in females. Spaying of females slows the growth of the transplanted tumors.
Another example of the relation of growth-stimulating hormones to tumor development has been proposed in diabetes type 2 and obesity. There is a doubling of the frequency of cancer in both conditions, which is attributed to excessive levels of insulin and insulin-like growth factors that are associated with insulin resistance (36).
The linear relationship between serum concentration and saturation density has long been known, but it was generally believed that it was only a physiological response. Such response would be expected to be reversible upon returning the cells to a single low concentration of serum, but it was shown that the relationship was partly inheritable when the cells were returned to serial passage at a single low concentration of serum and time interval (10). It also accelerated the progression to neoplastic development in transformed foci (Fig. 2). Increasing the time period of the 1° assay also accelerated the increases in saturation density and the rate of transformation in the serial rounds of selection at high cell density. The combination of these results indicates that even small increases in growth factors, such as insulin and stilbestrol, could produce significant increases in tumor development if maintained over sufficient periods of time.
The increasing permissiveness of cell populations for neoplastic progression with repeated rounds of selection or protracted periods of time needs to be considered in the likelihood of recurrence after surgical excision of tumors (34). It indicates the need to identify the area of preneoplastic fields around tumors to be included in such excision (37). It also suggests application of preventive treatments after tumor removal. That may include dietary changes, such as restriction of calories (38) and treatment with chemotherapeutic agents.
There is a long history of controversy whether genetic instability or selection is the driving force in tumor development (39, 40). The controversy has been mainly at the theoretical level. Our results at the simple experimental level under controlled environmental conditions, which had been optimized for the growth of NIH 3T3 cells under maximum definition, have made possible the dynamic cell population experiments that show that selection at confluence is the major driving force of neoplastic development. That conclusion is strengthened by the finding that transformation can be induced in cells growing at low population densities by reducing the concentration of CS from the standard 10% to 2% and passaging them frequently (16). The large reduction of CS allows selection of those cells from a heterogeneous population, which has a high fitness for growth at low serum concentrations.
There is a third method for transforming cells that supports selection as the driving force in the process. Suspending nontransformed BALB 3T3 cells in soft agar allowed formation of small colonies (≤0.2 mm) in 0.05% of the population and large transformed colonies (≥1.0 mm) in 0.005% of the population (41). The smaller colonies were morphologically different on plastic from the control, and from each other, with a wide range of growth rates. The large transformed colonies in 0.005% of the population differed in size and growth rate from each other. The changes related to those of epigenetic embryonic differentiation rather than that of genetic instability (42). Epigenetic selection indicates a major role for microenvironmental change in neoplastic development requiring an approach to prevention and treatment apart from the conventional killing of cancer cells. Mice provided by and used at California State Department of Health.
Supplementary Material
Acknowledgments
I appreciate the thoughtful comments of Dorothy Rubin in preparing the manuscript. The comments of Prof. Wallace McKeehan and Dr. Allen Mayer were insightful and greatly appreciated.
Footnotes
The author declares no conflict of interest.
References
- 1.Jainchill JL, Aaronson SA, Todaro GJ. Murine sarcoma and leukemia viruses: Assay using clonal lines of contact-inhibited mouse cells. J Virol. 1969;4:549–553. doi: 10.1128/jvi.4.5.549-553.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Varmus HE. The molecular genetics of cellular oncogenes. Annu Rev Genet. 1984;18:553–612. doi: 10.1146/annurev.ge.18.120184.003005. [DOI] [PubMed] [Google Scholar]
- 3.Graham FL, van der Eb AJ. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973;52:456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- 4.Shih C, Shilo B-Z, Goldfarb MP, Dannenberg A, Weinberg RA. Passage of phenotypes of chemically transformed cells via transfection of DNA and chromatin. Proc Natl Acad Sci USA. 1979;76:5714–5718. doi: 10.1073/pnas.76.11.5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Copeland NG, Zelenetz AD, Cooper GM. Transformation of NIH/3T3 mouse cells by DNA of Rous sarcoma virus. Cell. 1979;17:993–1002. doi: 10.1016/0092-8674(79)90338-6. [DOI] [PubMed] [Google Scholar]
- 6.Perucho M, et al. Human-tumor-derived cell lines contain common and different transforming genes. Cell. 1981;27:467–476. doi: 10.1016/0092-8674(81)90388-3. [DOI] [PubMed] [Google Scholar]
- 7.Krontiris TG, Cooper GM. Transforming activity of human tumor DNAs. Proc Natl Acad Sci USA. 1981;78:1181–1184. doi: 10.1073/pnas.78.2.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shih C, Padhy LC, Murray M, Weinberg RA. Transforming genes of carcinomas and neuroblastomas introduced into mouse fibroblasts. Nature. 1981;290:261–264. doi: 10.1038/290261a0. [DOI] [PubMed] [Google Scholar]
- 9.Shipley GD, Ham RG. Improved medium and culture conditions for clonal growth with minimal serum protein and for enhanced serum-free survival of Swiss 3T3 cells. In Vitro. 1981;17:656–670. doi: 10.1007/BF02628401. [DOI] [PubMed] [Google Scholar]
- 10.Rubin H. Incipient and overt stages of neoplastic transformation. Proc Natl Acad Sci USA. 1994;91:12076–12080. doi: 10.1073/pnas.91.25.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aaronson SA, Todaro GJ. Basis for the acquisition of malignant potential by mouse cells cultivated in vitro. Science. 1968;162:1024–1026. doi: 10.1126/science.162.3857.1024. [DOI] [PubMed] [Google Scholar]
- 12.McKeehan WL, Ham RG. Stimulation of clonal growth of normal fibroblasts with substrata coated with basic polymers. J Cell Biol. 1976;71:727–734. doi: 10.1083/jcb.71.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubin H, Xu K. Evidence for the progressive and adaptive nature of spontaneous transformation in the NIH 3T3 cell line. Proc Natl Acad Sci USA. 1989;86:1860–1864. doi: 10.1073/pnas.86.6.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubin H. Promotion and selection by serum growth factors drive field cancerization, which is anticipated in vivo by type 2 diabetes and obesity. Proc Natl Acad Sci USA. 2013;110:13927–13931. doi: 10.1073/pnas.1312831110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubin H. Microenvironmental regulation of the initiated cell. Adv Cancer Res. 2003;90:1–62. doi: 10.1016/s0065-230x(03)90001-7. [DOI] [PubMed] [Google Scholar]
- 16.Rubin AL, Yao A, Rubin H. Relation of spontaneous transformation in cell culture to adaptive growth and clonal heterogeneity. Proc Natl Acad Sci USA. 1990;87:482–486. doi: 10.1073/pnas.87.1.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubin AL, Arnstein P, Rubin H. Physiological induction and reversal of focus formation and tumorigenicity in NIH 3T3 cells. Proc Natl Acad Sci USA. 1990;87:10005–10009. doi: 10.1073/pnas.87.24.10005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shipley GD, Ham RG. Attachment and growth of Swiss and Balb/c3T3 cells in a completely serum-free medium. In Vitro. 1980;16:218. [Google Scholar]
- 19.Aaronson SA, Todaro GJ. Development of 3T3-like lines from Balb-c mouse embryo cultures: Transformation susceptibility to SV40. J Cell Physiol. 1968;72:141–148. doi: 10.1002/jcp.1040720208. [DOI] [PubMed] [Google Scholar]
- 20.Grundel R, Rubin H. Maintenance of multiplication rate stability by cell populations in the face of heterogeneity among individual cells. J Cell Sci. 1988;91:571–576. doi: 10.1242/jcs.91.4.571. [DOI] [PubMed] [Google Scholar]
- 21.Rubin H. Early origin and pervasiveness of cellular heterogeneity in some malignant transformations. Proc Natl Acad Sci USA. 1984;81:5121–5125. doi: 10.1073/pnas.81.16.5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Temin HM, Rubin H. Characteristics of an assay for Rous sarcoma virus and Rous sarcoma cells in tissue culture. Virology. 1958;6:669–688. doi: 10.1016/0042-6822(58)90114-4. [DOI] [PubMed] [Google Scholar]
- 23.Prince AM. Quantitative studies on Rous sarcoma virus. III. Virus multiplication and cellular response following infection of the chorioallantoic membrane of the chick embryo. Virology. 1958;5:435–457. doi: 10.1016/0042-6822(58)90038-2. [DOI] [PubMed] [Google Scholar]
- 24.Rubin H. The inhibition of chick embryo cell growth by medium obtained from cultures of Rous sarcoma cells. Exp Cell Res. 1966;41:149–161. doi: 10.1016/0014-4827(66)90555-6. [DOI] [PubMed] [Google Scholar]
- 25.Rubin H. The suppression of morphological alterations in cells infected with Rous sarcoma virus. Virology. 1960;12:14–31. doi: 10.1016/0042-6822(60)90146-x. [DOI] [PubMed] [Google Scholar]
- 26.Farber E. The multistep nature of cancer development. Cancer Res. 1984;44:4217–4223. [PubMed] [Google Scholar]
- 27.Tsao J-L, et al. Genetic reconstruction of individual colorectal tumor histories. Proc Natl Acad Sci USA. 2000;97:1236–1241. doi: 10.1073/pnas.97.3.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heisenberg W. Philosophical Problems of Quantum Physics. Ox Bow; Woodbridge, CT: 1979. [Google Scholar]
- 29.Stratton MR. Exploring the genomes of cancer cells: Progress and promise. Science. 2011;331:1553–1558. doi: 10.1126/science.1204040. [DOI] [PubMed] [Google Scholar]
- 30.Martincorena I, et al. High burden and pervasive positive selection of somatic mutations in normal human skin. Science. 2015;348:880–886. doi: 10.1126/science.aaa6806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friedewald WF, Rous P. The initiating and promoting elements in tumor production: An analysis of the effects of tar, benzpyrene, and methylcholanthrene on rabbit skin. J Exp Med. 1944;80:101–126. doi: 10.1084/jem.80.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Todaro GJ, Green H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Willis RA. The mode of origin of tumors: Solitary localized squamous cell growths of the skin. Cancer Res. 1944;4:630–644. [Google Scholar]
- 34.Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium: Clinical implications of multicentric origin. Cancer. 1953;6:963–968. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 35.Foulds L. Neoplastic Development. Vol 1 Academic; New York: 1969. [Google Scholar]
- 36.Giovannucci E. Insulin, insulin-like growth factors and colon cancer: A review of the evidence. J Nutr. 2001;131(11 Suppl):3109S–3120S. doi: 10.1093/jn/131.11.3109S. [DOI] [PubMed] [Google Scholar]
- 37.Poh CF, et al. Fluorescence visualization detection of field alterations in tumor margins of oral cancer patients. Clin Cancer Res. 2006;12:6716–6722. doi: 10.1158/1078-0432.CCR-06-1317. [DOI] [PubMed] [Google Scholar]
- 38.Bruce WR, Giacca A, Medline A. Possible mechanisms relating diet and risk of colon cancer. Cancer Epidemiol Biomarkers Prev. 2000;9:1271–1279. [PubMed] [Google Scholar]
- 39.Tomlinson IPM, Novelli MR, Bodmer WF. The mutation rate and cancer. Proc Natl Acad Sci USA. 1996;93:14800–14803. doi: 10.1073/pnas.93.25.14800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schöllnberger H, Beerenwinkel N, Hoogenveen R, Vineis P. Cell selection as driving force in lung and colon carcinogenesis. Cancer Res. 2010;70:6797–6803. doi: 10.1158/0008-5472.CAN-09-4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rubin H. Uniqueness of each spontaneous transformant from a clone of BALB/c 3T3 cells. Cancer Res. 1988;48:2512–2518. [PubMed] [Google Scholar]
- 42.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]