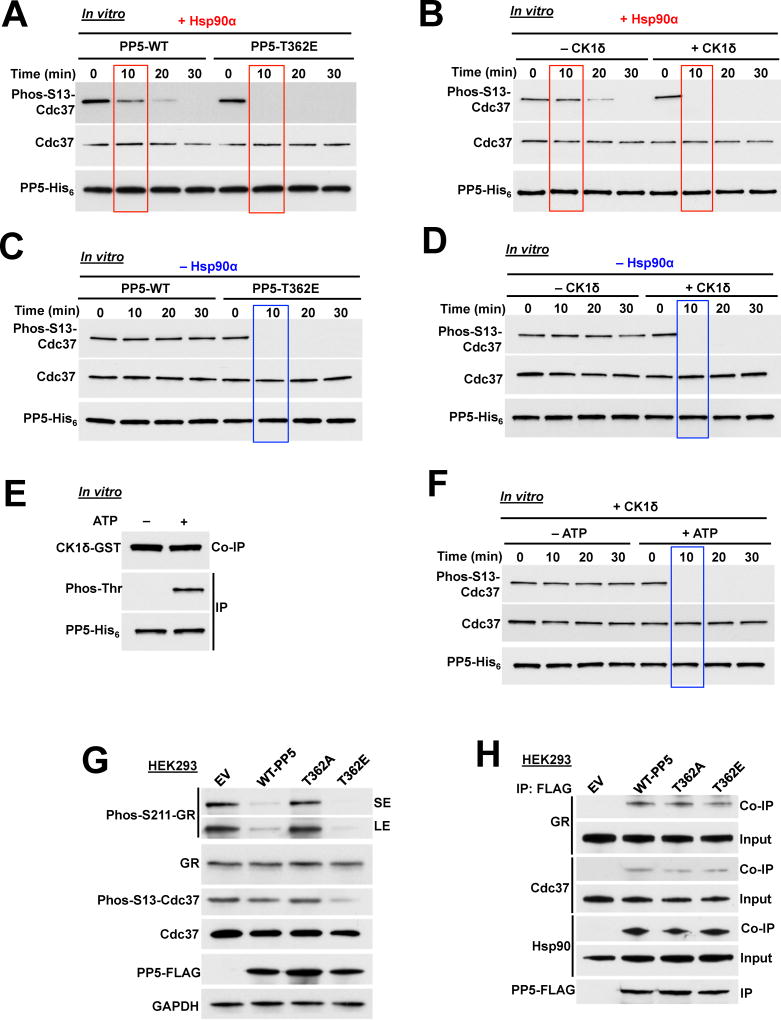
Figure 2. CK1δ mediated phosphorylation of PP5 activates and increases the rate of phosphatase activity. See also Figure S2.
A) Dephosphorylation of phospho-S13-Cdc37 with a recombinant wild-type PP5-His6 and phosphomimetic T362E-PP5-His6, in the presence of Hsp90α. Rate of Cdc37 dephosphorylation was assessed by immunoblotting with a phospho-specific S13-Cdc37 antibody over time (minutes).
B) Recombinant wild-type PP5-His6 was phosphorylated by CK1δ in vitro and then used in the dephosphorylation of phospho-S13-Cdc37 in vitro. The assay was performed in presence of Hsp90α. PP5 activity was assessed with immunoblotting using a phospho-specific S13-Cdc37 antibody over time (minutes).
C) Dephosphorylation of phospho-S13-Cdc37 with recombinant wild-type PP5-His6 and phosphomimetic T362E-PP5-His6 was performed in the absence of Hsp90α. Activity was assessed with immunoblotting using a phospho-specific S13-Cdc37 antibody over time (minutes).
D) Recombinant wild-type PP5-His6 was phosphorylated by CK1δ in vitro and then used in the dephosphorylation of phospho-S13-Cdc37 in vitro without Hsp90α. PP5 activity was assessed with immunoblotting using a phospho-specific S13-Cdc37 antibody over time (minutes).
E) Recombinant PP5-His6 was used in an in vitro kinase assay with CK1δ-GST. PP5-His6 was immunoprecipitated (IP) and threonine phosphorylation of PP5 as well as co-immunoprecipitation (Co-IP) of CK1δ-GST were examined by immunoblotting with anti-phosphothreonine and anti-GST antibodies.
F) Recombinant wild-type PP5-His6 was phosphorylated by CK1δ in vitro in the presence (+) or absence (−) of ATP. PP5-His6 proteins were then used in the dephosphorylation of phospho-S13-Cdc37 in vitro without Hsp90α. PP5 activity was assessed with immunoblotting using a phospho-specific S13-Cdc37 antibody over time (minutes).
G) PP5-FLAG and T362-PP5 phosphomutants (T362A and T362E) were transiently transfected in HEK293 cells. Cdc37, phospho-S13-Cdc37, GR and phospho S211-GR protein levels were examined by immunoblotting. Empty vector (EV) was used as a control, GAPDH was used a loading control.
H) Wild-type PP5-FLAG, non-phosphorylating T362A-PP5-FLAG and the phosphomimetic T362E-PP5-FLAG were transiently expressed and IP from HEK293 cells. Co-IP of GR, Cdc37 and Hsp90 were examined by immunoblotting.