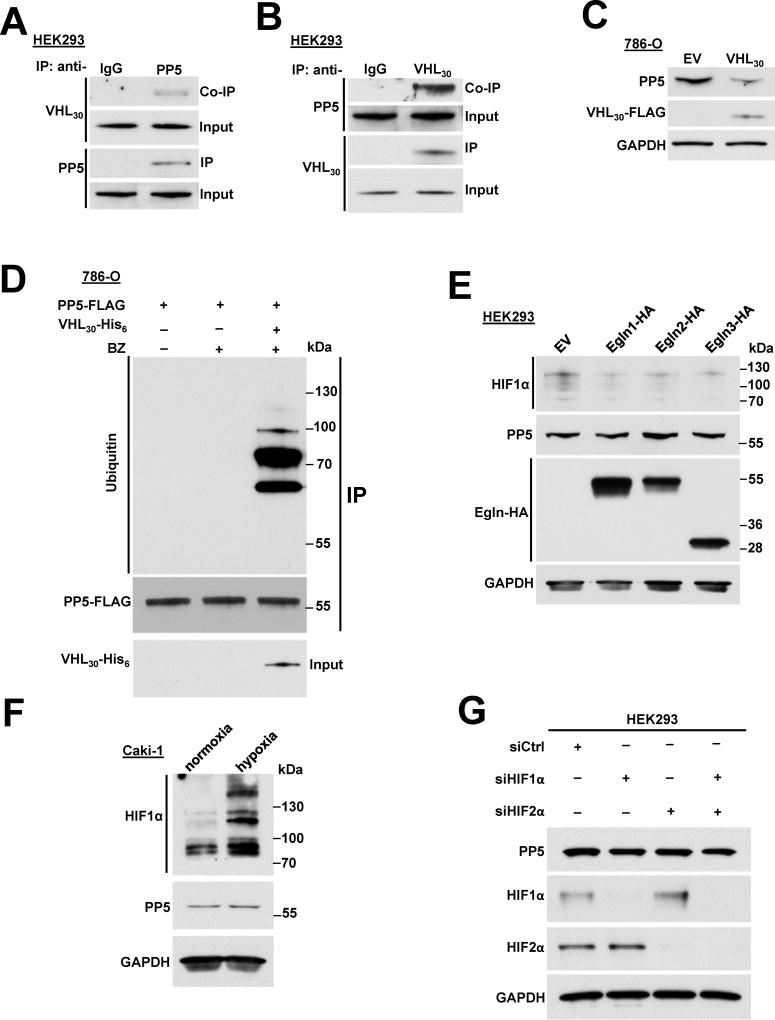
Figure 3. VHL E3 ligase ubiquitinates PP5 independent of hypoxia. See also Figure S3.
A) Endogenous PP5 was immunoprecipitated (IP) from HEK293 cells and co-immunoprecipitation (Co-IP) of VHL30 was assessed by immunoblotting.
B) Endogenous VHL30 was IP from HEK293 cells and Co-IP of PP5 was examined by immunoblotting.
C) VHL30-FLAG or empty vector (EV) was transiently over-expressed in 786-O cells and endogenous PP5 protein levels were assessed by immunoblotting. GAPDH was used a loading control.
D) The 786-O cells transiently expressing PP5-FLAG, were treated with or without 50 nM proteasome inhibitor bortezomib (BZ) for 2 hr. PP5-FLAG was also co-expressed with VHL30-His6 with additional treatment of 50 nM BZ for 2 hr. PP5-FLAG was IP and its ubiquitination was assessed by immunoblotting.
E) Egln1-HA, Egln2-HA, Egln3-HA and empty vector pcDNA3.1 were transiently over-expressed in HEK293 cells. The expression of Egln1, 2 and 3 as well as PP5, and HIF1α were assessed by immunoblotting with anti-HA, anti-PP5 and anti- HIF1α antibodies. GAPDH was used a loading control.
F) Caki-1 cells cultured in normoxia and hypoxia (1%O2, 5%CO2, 94%N2). PP5 and HIF1α protein levels were examined by immunoblotting using anti-PP5 and anti-HIF1α antibodies. GAPDH was used a loading control.
G) HIF1α or HIF2α were silenced by small interfering RNA (siRNA) in HEK293 cells. HIF1α, HIF2α and PP5 protein levels were examined by immunoblotting using anti-HIF1α, anti-HIF2α and anti-PP5 antibodies. GAPDH was used a loading control.