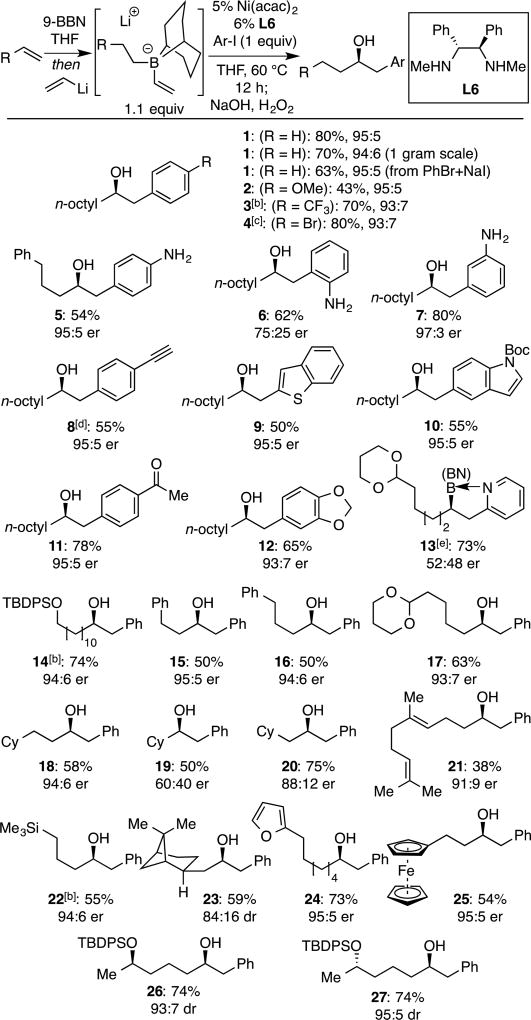
Table 2.
Scope of Ni-Catalyzed Enantioselective Conjunctive Coupling[a]
See text and Supporting Information details. Vinyllithium prepared from n-BuLi and tetravinyl stannane. Yields are of purified material and represent the average value for two experiments.
Vinyllithium prepared from n-BuLi and vinyl iodide.
Reaction conducted at room temperature.
Substrate was (4-iodophenyl)triethylsilyl acetylene, which undergoes desilylation during oxidation.
Product difficult to oxidize; isolated as the borane after silica gel chromatography.