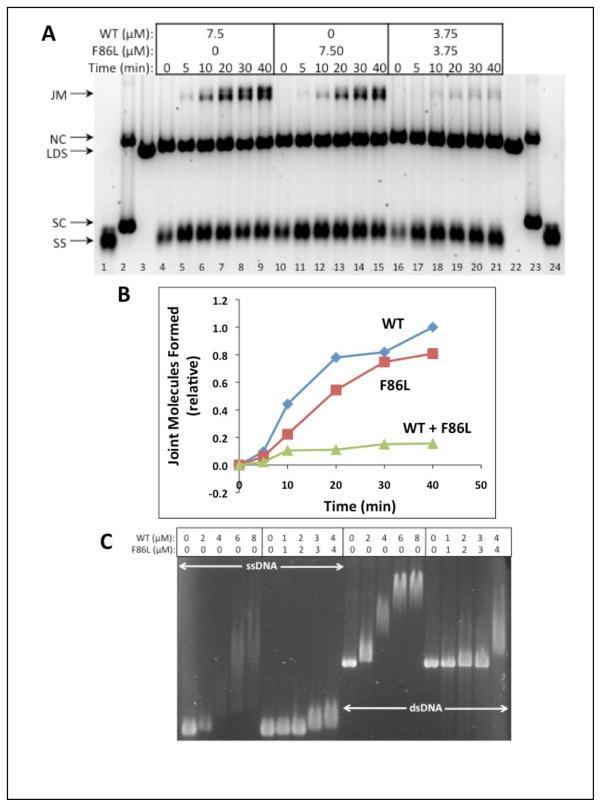
Figure 10. DNA strand exchange activity of RAD51 F86L versus WT with homologous ϕX174 DNA substrates and RPA protein.
(A) Reactions were carried out as described in Materials and Methods and as shown schematically in Figure 9A. Timecourses of reactions containing a total of 7.50 μM RAD51 protein (WT and/or F86L) at the indicated concentrations. The positions of SS, LDS, NC, and SC (supercoiled ϕX174 DNA) markers (lanes 1–3, 22–24) are indicated next to the gel, along with the position of JM products. Left (lanes 4–9)-- Reaction with WT only; Center (lanes 10–15)-- Reaction with F86L only; Right (lanes 16–21)-- Reaction with 1:1 mixture of WT and F86L. (B) Quantitative analysis of joint molecule formation based on image scans were performed as described in Materials and Methods. Data are normalized relative to the total density of the Joint Molecules products formed by wild-type RAD51 at the 40 min time point. Results in panels (A) – (B) are typical of those obtained in three separate experiments performed under identical conditions. (C) Electrophoretic mobility shift assays of mixed RAD51 WT/F86L versus WT complexes formed with circular ϕX174 ssDNA (lanes 1–10) and linear ϕX174 dsDNA (lanes 11–20). Experimental conditions were identical to those in Fig. 5C–D except that in lanes 6–10 and in lanes 16–20 the DNA molecules were titrated with a 1:1 mixture of RAD51 WT and F86L proteins.