Abstract
Background
Histologic prognostic factors have been described for nodular lymphocyte predominant Hodgkin lymphoma (NLPHL). This study examines histologic and immunophenotypic variants in a clinical trial for pediatric NLPHL.
Procedure
One hundred sixty-eight cases of localized NLPHL were examined for histologic variants, CD30 and immunoglobulin D (IgD) expression, and outcome. Histologic types were scored categorically as 0 = 0, 1 ≤ 25%, and 2 > 25% of the sample.
Results
Fifty-eight (35.1%) cases showed only typical nodular with or without serpiginous histology (types A and B). The remainder showed mixtures of histologies. The numbers of patients with score 2 are 85 (50.6%) type A, 21 (12.5%) type B, 46 (27.4%) with extranodular large B cells (type C), 3 with T-cell-rich nodular pattern (type D), 55 (32.7%) with diffuse T-cell-rich (type E) pattern, and 2 (1.2%) with diffuse B-cell pattern (type F). Higher level of types C (P = 0.048) and D (P = 0.033) resulted in lower event-free survival (EFS). Cytoplasmic IgD was found in 65 of 130 tested (50%), did not significantly associate with EFS but positively correlated with types C and E histology (P < 0.0001) and negatively correlated with types A (P = 0.0003) and B (P = 0.006). Seventeen (10%) expressed CD30, with no adverse effect.
Conclusions
Variant histology is common in pediatric NLPHL, especially types C and E, which are associated with IgD expression. Type C variant histology and possibly type D are associated with decreased EFS, but neither IgD nor CD30 are adverse features. Variant histology may warrant increased surveillance, but did not affect overall survival.
Keywords: children, histology, Hodgkin, lymphoma, pathology
1 INTRODUCTION
Nodular lymphocyte predominant (LP) Hodgkin lymphoma (NLPHL) represents 5–10% of Hodgkin lymphoma (HL) with a male predominance and typical presentation of low-stage disease without B symptoms. NLPHL is usually slow-growing and responsive to therapy, but may be more aggressive.1
Children’s Oncology Group trial AHOD03P1 was designed to test the efficacy of stage-directed reduced-therapy treatment of low-risk NLPHL in young people, with stage IA or IIA disease.1 At the time of protocol design, there had been recent reports of a relatively new subtype of classical HL (CHL) mimicking NLPHL, lymphocyte-rich CHL.2 These were noted in one large series to constitute about 34% of cases previously diagnosed as NLPHL.3
The protocol was designed with a “watch and wait” component for totally resected stage IA disease present in a single node. Out of concern that this would not be appropriate for CHL, all cases were required to have immunohistochemical (IHC) staining performed, and all were subjected to rapid pathology review.
The morphology and immunohistochemistry of NLPHL show nodules of small B cells with admixed larger neoplastic LP cells (Fig. 1A).4,5 (LP cells are also sometimes referred to as lymphocytic and histiocytic (L&H) cells, from an earlier name of lymphocytic and histiocytic HL.) LP cells are mature B cells that label positively for CD20, CD45, B-cell transcription factors OCT2 and BOB1, and immunoglobulin J-chain. They are usually negative for both CD30 and CD15 (in contrast to classical Hodgkin–Reed–Sternberg cells that are typically positive for both). T cells often rosette around the large cells (Fig. 1B) and CD57/CD4-positive T cells may be increased in the nodules, while other T cells predominate in the internodular areas.
FIGURE 1.
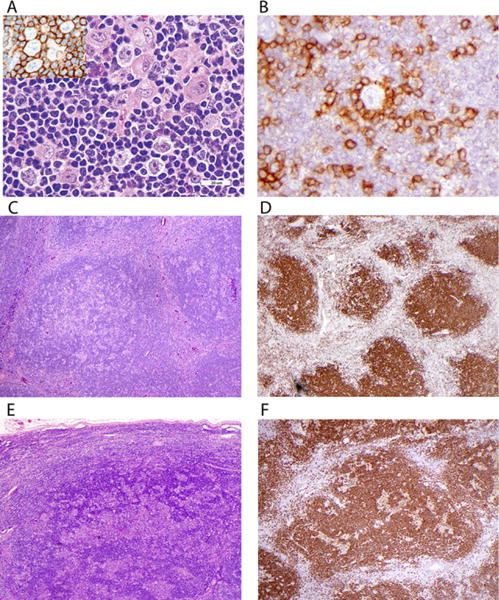
Histology and immunophenotype of typical nodular lymphocyte predominant Hodgkin lymphoma. (A) Scattered large LP cells are present, along with a few histiocytes, in a nodule of small B cells in typical NLPHL. The inset shows B-cell marker CD20 antibody labeling both small and large cells. (B) Small T cells labeled with anti-CD3 (illustrated here) and often CD57 form rosettes around the LP cells. (C) Nodules of small lymphocytes with admixed LP cells and histiocytes in typical type A histology. (D) CD20 shows the B-cell nature of nodules. (E) Some cases show a serpiginous growth pattern in type B NLPHL. (F) CD20 labels both small B cells and LP cells in serpiginous areas of type B NLPHL
Morphologic patterns have been described to include classic morphology (nodular or serpiginous, type A or B), as well as several categories of variant histology, including those with prominent extra-nodular LP cells (pattern C), T-cell-rich nodules (pattern D), diffuse T-cell-rich (T-cell-rich large B-cell lymphoma like, pattern E) and diffuse B-cell-rich patterns (moth-eaten, pattern F).6 Variant histology has been incorporated into novel grading schemes for NLPHL as it has been tentatively associated with adverse features.7–10
Positivity for CD30 has been noted in up to 28% of NLPHL, usually focal or heterogeneous and more frequent in the presence of Epstein–Barr virus (EBV).11–14 CD30 may provide a target for anti-CD30 ther apeutic antibodies in the rare CD30-positive cases that relapse.15
IgD expression has been described to be frequent in young male patients and to be associated with the presence of large B cells in the extranodular regions.7,16 In this study we examine the histologic patterns, CD30 and IgD expression of NLPHL, and compare them relative to patient outcomes in the context of a single pediatric clinical trial.
1.1 Patients and methods
1.1.1 Patient group
Patients were those enrolled in a nonrandomized clinical trial (study AHOD03P1) for low-risk NLPHL conducted at Children’s Oncology Group institutions from January 2006 through November 2010 (ClinicalTrials.gov Identifier NCT00107198). Written informed consent was obtained according to institutional guidelines and in compliance with Declaration of Helsinki guidelines. Eligible patients were less than 22 years of age with stage IA/IIA NLPHL. Patients with B symptoms or bulky disease were excluded from the study.
1.1.2 Histology
Stained and unstained slides from fixed, paraffin-embedded lymph node biopsies were histologically reviewed and assessed according to histologic pattern, and according to CD20, CD30, and IgD immunophenotype of the neoplastic LP cells. CD20 and CD30 were performed prospectively at treating institutions and were required for entry on the protocol. Protocol guidelines also recommended a minimum antibody panel of CD45, CD3 (or CD45Ro), CD20, CD15, CD30, and kappa and lambda immunoglobulins, with optional recommendation of CD57, BOB.1, EMA, CD79a, BCL6, CD68, Oct2, ALK-1, and J-chain. CD21 and/or CD23 were scored in some cases, although not stressed for diagnosis at the time of protocol design. Most cases had been vetted by expert hematopathologists prior to enrollment in the study. All submitted slides were reviewed prospectively and diagnoses confirmed on enrollment in a rapid review.
Submitted CD30 stains were examined, staining of all cases described as positive at the treating institution was repeated, and additionally was repeated for all other cases with unstained slides. Final scoring of CD30 was based on our review of one or more slides. For most cases, CD20 staining was also repeated. IgD was performed on all cases that had available unstained slides. Repeat IHC stains were performed on an automated immunostainer (Ventana Medical Systems, Tucson AZ), with monoclonal antibodies CD30, CD20, CD3, and anti-IgD (Cell Marque, Rocklin, CA) using standard technique.
All cases were categorized based on morphology into five categories, corresponding to the patterns reported by Fan et al.6 Each case was assessed for percent involvement by each histologic type, and then condensed to a score of 0–2 as described in the statistical section.
1.1.3 Treatment strategy
Patients with stage I disease and total resection of a single involved node were observed without further therapy; recurrences were treated with three cycles of AV-PC (doxorubicin/vincristine/prednisone/cyclophosphamide). Patients with unresected stage IA or stage IIA were treated with three cycles of AV-PC. Patients with less than a complete response (CR) to AV-PC received 21 Gy involved-field radiation therapy.1
1.1.4 Statistics
For each histologic type, A–E, the proportion of involvement of available sample was scored 0–2: 0 = 0, 1 < 25%, 2 > 25% for variant histology. CD30 and IgD expression was divided into two groups based on the groupings (yes/no). Statistical inference (Chi-square test on categorical variables and t-test on continuous variables) was performed to compare the demographics between groups. The event-free survival (EFS) curves were compared among the three levels of each variant histology, and between the two levels of CD30 and IgD expression by log-rank tests. Polychoric or tetrachoric correlations were also measured among variant histology, CD30 and IgD expression.
2 RESULTS
Details of patient characteristics, therapy, and overall clinical outcomes have been previously reported.1 Briefly, 188 patients were enrolled, 183 were eligible, and 178 were evaluable for analysis in the clinical report of this protocol; 5 patients who had protocol deviations were excluded. Among these, 168 cases were available for further histopathologic evaluation at the end of protocol (slides were no longer available in 10 cases). The mean age at enrollment was 12.3 years (range 4.2–20.7), and 85% were males. The most common sites of involvement were lymph node groups in the head and neck, axilla, or groin. Mediastinal involvement was rare (n = 7, 3.8%). A diagnosis of NLPHL was confirmed by central review for each case. EFS at 5 years was 85.5% and overall survival (OS) at 5 years was 100%.
Fifty-nine (35.1 %) cases showed only typical nodular or nodular and serpiginous histology (types A and B; Fig. 1 C–F). The remainder showed mixtures of typical and variant histologies. Only categorical scores of 2 are described in this section unless noted otherwise, since no scores of 1 showed adverse outcome. The numbers of patients who have score 2 are as follows: 85 (50.6%) type A, 21 (12.5%) type B, 46 (27.4%) with extranodular large B cells (type C, Fig. 2A), 3 with T-cell-rich nodular pattern (type D, Figs. 2B and 2C), 55 (32.7%) with diffuse T-cell-rich (type E, Fig. 2D) pattern, and 2 (1.2%) with diffuse B-cell moth-eaten pattern (type F, Fig. 2E). There was a total of five patients (three patients with score 1 and two patients with score 2) with type F, and these were younger than other patients, 5.0 ± 0.8 and 5.0 ± 0.4 years, respectively, versus 12.3 ± 4.0 years for other patients, P = 0004. Type C was associated with decreased EFS (Fig. 3C), as was type D (Fig. 3D), although there were few patients with D (7 patients with score 1 and 3 patients with score 2; only score 2 showed decreased survival). Cases with both types C and E were frequent (Fig. 2F).
FIGURE 2.
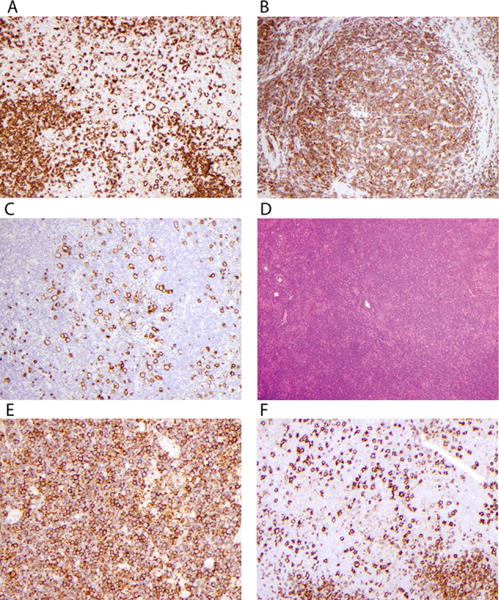
Examples of variant histology. (A) Fan type C histology shows large cells labeling with anti-CD20 in extranodular areas. (B) Small lymphocytes in nodules of this case label with anti-CD3, identifying type D NLPHL. (C) CD20 highlights LP cells in the CD20-negative T-cell background of nodules in type D. (D) This hematoxylin and eosin (H&E) stain shows an apparently diffuse infiltrate in a case with type E morphology. (E) This case has >25% with diffuse small lymphocytes labeling with anti-CD20 as diffuse B-cell type D. This case is not as “moth-eaten” as usually described. (F) This case labeled with anti-CD20 shows type C with increased extranodular large B cells, merging into a diffuse type E pattern
FIGURE 3.
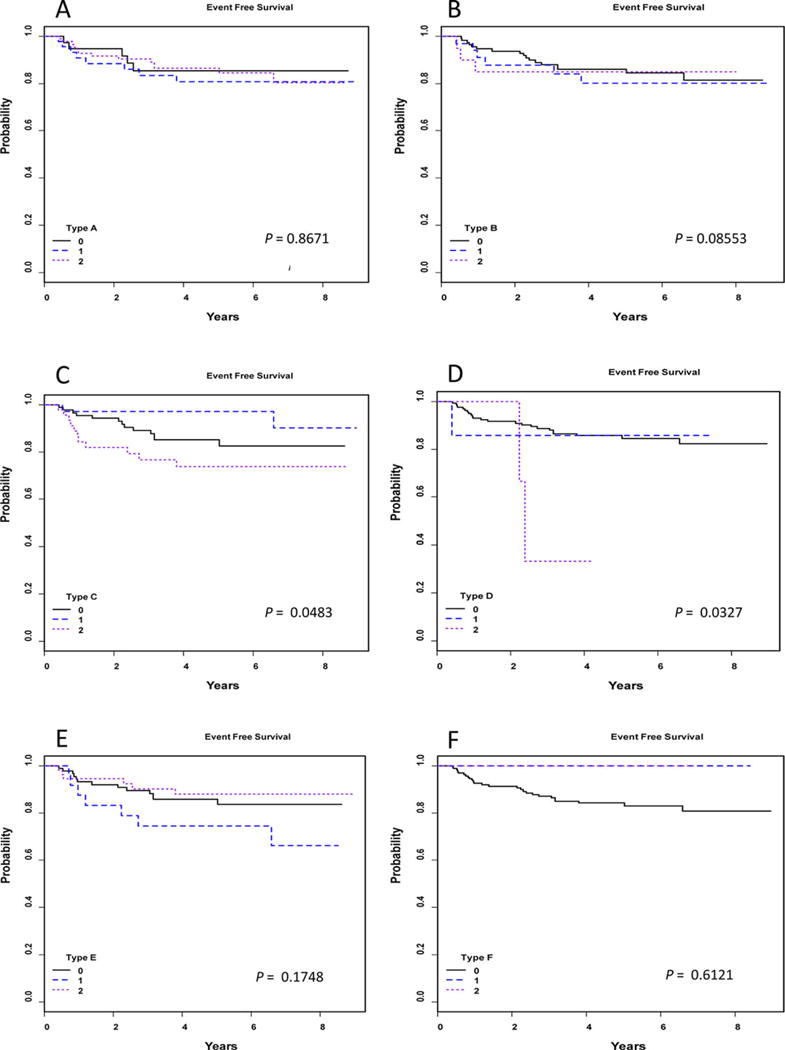
Survival curves. Kaplan–Meier survival curves for each histologic variant A–F show categorical scores of 0, 1, and 2, with significant differences seen in type C with extranodular LP cells and type D, nodular T-cell-rich type, each showing decreased EFS with category 2
IgD expression was found in LP cells in 65 of 130 tested (50%) (Fig. 4A), was not associated with outcome, but was positively correlated with types C and E histology (P < 0.0001), and negatively correlated with types A (P = 0.003) and B (P = 0.0059) (Fig. 5).
FIGURE 4.
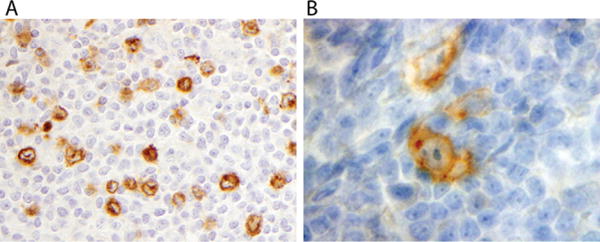
(A) Extranodular LP cells show cytoplasmic IgD staining in a background of small T cells in this case that includes score 2 types C and E, along with score 1 type A; (B) LP cells labels with anti-CD30
FIGURE 5.
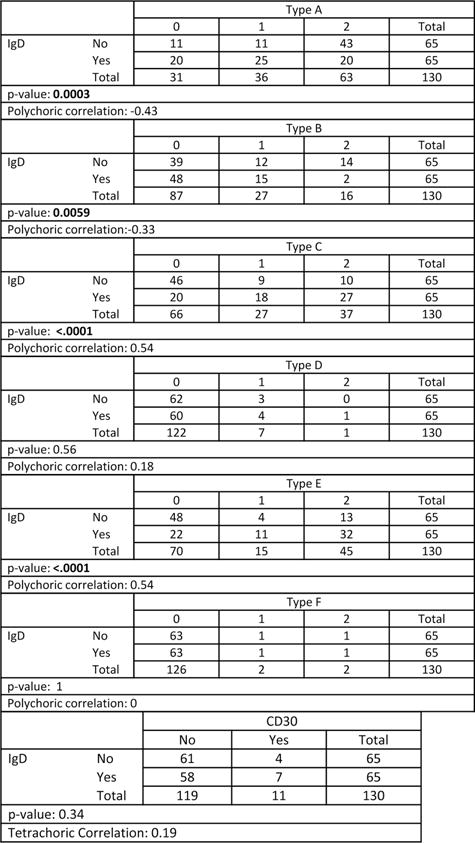
Categorical scores for each histologic type and for CD30 are compared for IgD expression. Note. Cytoplasmic IgD is negatively correlated with histologic types A (P = 0.0003) and B (P = 0.0059), and is positively correlated with types C and E (P < 0.0001).
Seventeen of 168 cases (10.1%) showed CD30 positivity (Fig. 4B). Of these, 10 had adequate slides for EBV-encoded ribonucleotide (EBER) in situ hybridization (ISH). One (10% of the CD30+ cases that were tested) showed EBER positivity in a pattern consistent with clonal integration. There was no difference in EFS based on CD30 expression.
In sum, variant histology is common in pediatric NLPHL, especially types C and E, which are associated with IgD expression. Type C variant histology and possibly type D are associated with decreased EFS, but neither IgD nor CD30 expression are adverse features. OS could not be compared since all patients survived.
3 DISCUSSION
NLPHL is an uncommon disease that is frequently indolent and often cured with chemotherapy and/or RT regimens designed for CHL. Such treatment, however, poses long-term risks and efforts have been made to reduce therapy in children while preserving good therapeutic outcomes. This is particularly important in NLPHL, since some patients have been cured with local therapy such as resection alone. COG protocol AHOD03P1 tested whether excellent outcomes could be preserved in patients with low-risk NLPHL by resection alone or minimal chemotherapy, and the results appear to be very good.1
Some patients with NLPHL may demonstrate a more aggressive course than usual, with relapse following therapy or transformation to B-cell non-Hodgkin lymphoma; thus, efforts have been made to identify prognostic factors.17,18 Presence or absence of CD30 expression has been utilized to help distinguish between NLPHL and CHL, variation in histology has been studied, and IgD expression has also been examined.6,7,9,16
A few studies have noted that some cases of NLPHL express CD30, usually in weak and/or focal patterns, and that the morphology and immunophenotype of these is similar to CD30-negative cases.11,12 A rare case of relapsed NLPHL with CD30 expression showed response to brentuximab vedotin, a chimeric anti-CD30 monoclonal antibody conjugate that is widely used for refractory CHL.15 Huppmann et al. in a large study of adult and pediatric NLPHL, reported that 3–5% expressed EBER transcripts and that these more frequently expressed CD30 than did EBV-negative cases.13 In our current study, only one CD30-positive case was found to express EBER. CD30-negative cases were not tested for EBER.
The earliest description of variant histology in lymphocyte predominance Hodgkin lymphoma (LPHL; the accepted term at that time) is from Dennis Wright’s group at Southampton, UK, that studied 57 cases with a panel of monoclonal antibodies in 1989.4 Fifteen cases showed diffuse histology, most of which were reclassified as CHL, splenic B-cell lymphoma, peripheral T-cell lymphoma, or reactive hyperplasia. Four cases were classified as “diffuse lymphocyte predominance” HL and six additional cases were categorized as LPHL with indistinct nodularity. Diffuse cases and cases with indistinct nodularity lacked both CD15 and CD30 and showed polylobated B cells against a background of small T lymphocytes in the extranodular areas, which was shown in illustration, and each showed a rim of unstained T lymphocytes surrounding the polylobated cells on CD20 stain.
The WHO classification of hematopoietic neoplasms, first published in 2001 (updated in 2008), added the word “nodular” to the diagnosis of LPHL and defined NLPHL to require at least a partially nodular pattern.2 In 2003, Fan et al. analyzed 137 samples from 118 patients with NLPHL and described the six patterns utilized in this study.6 Histologic subtypes overlapped in their study and there were no cases of pure type C or pure diffuse type E. Our findings are similar in these regards. In their study, 16 cases were predominantly diffuse while 19 showed a minor component of diffuse (total = 35). Ten cases showed a predominant pattern of type C and 41 showed presence of a minor component (total = 51). Thus, 43% showed some type C pattern, compared to 63% of our pediatric cases. Most samples (127 of 137) showed some component of classic nodular type A, while 54 showed only type A. Patients with and without recurrence were compared. Samples of those with no recurrence (n = 27) had median age of 46 years (range 6–91) and a median follow-up of 20 months. Those patients with recurrence (n = 27) had a median age of 31 (range 4–60) and median follow-up of 24 months. Diffuse pattern, both predominant (P = 0.001) and presence of diffuse (P = 0.003), correlated with recurrence but no other pattern did. Cases with multiple specimens showed a trend for cases with type C to develop diffuse pattern and for cases with diffuse pattern to relapse as diffused large B-cell lymphoma (DLBCL; five cases). In some cases, diffuse components were not persistent at relapse.
Hartmann et al. studied 423 cases of NLPHL (ages not defined).19 Three hundred fifty (82.7%) had either typical nodular histology (A) or serpiginous type (B), and 105 (25.4%) had one of the other variants described by Fan et al. Variants had higher stage, higher international prognostic index (IPI) score and less 5-year progression-free survival (P = 0.0158), but no difference in overall survival. The authors also found that low serum albumin and male gender were adverse features, and described a prognostic scoring system using variant histology, albumin, and gender.
A study by Shankar et al. of pediatric NLPHL also found that variant histology was associated with higher stage at diagnosis, lower CR rates, and higher relapse.9 Additionally, IgD expression correlated with poorer treatment response and was more common in patients with variant histology. That study included 60 patients, all 18 years old or less, predominantly with stage IIA disease and 6 (10%) with stage IIIA. Twenty-three percent were treated with surgery alone, 67% with CVP chemotherapy, and 10% with other chemotherapy. There was a notably lower proportion of type C in their study than in our study, with most variants being diffuse (23% total variant histology).
Compared to our results that found IgD expression in 64 of 130 cases (49%), a study by Prakash et al. identified 27% IgD-positive patients.16 Median age in that study was 38 years (range 7–81 years). They demonstrated that IgD expression was associated with younger age, male predominance, cervical lymph node involvement, and prominent extranodular localization of LP cells (type C). Recently, Hartmann et al. also evaluated the prognostic role of several additional histologic features in NLPHL and showed no association between IgD and outcome.7 Seven of eight IgD-positive cases showed type C histology. Variant histology in NLPHL is currently being included in an updated revision of the WHO classification, based on the findings of association with advanced disease and relapse.20
In our study, the most frequent histologic types were A, B, C, and E, and all combinations of these (Fig. 2F and Fig. 6). Types C and E are both associated with cytoplasmic IgD expression. Cases with type C also tended to show smaller nodules of small B cells compared to type A. This raises the possibility that cases begin with type A (with or without B), that the nodules of small B cells regress with development of areas of type C, which then further evolve to diffuse type E. The role of cytoplasmic IgD is unknown, although surface IgD is attributed to the origin of nodules from the surface of IgD- and IgM-positive mantle zone cells.3 While the proposed evolution is a conjecture, it was also noted by Fan et al. that there is a tendency for progression of NLHPL over time to diffuse involvement, that type C may be an early form of this progression, and that NLPHL and DLBCL may be related.6 Xing et al. argue that the outcome of NLPHL is largely stage-related and that therapy usually applied to DLBCL may be optimal for relapsed NLPHL.21 In their study, relapses of NLPHL included cases of usual DLBCL and also T-cell/histiocyte-rich large B-cell lymphoma, which resembles the diffuse areas of type E NLPHL. Studies of low-risk NLPHL in children with long follow-up do not, however, show any advantage of increased therapy or increased incidence of evolution to DLBCL.18,22
FIGURE 6.
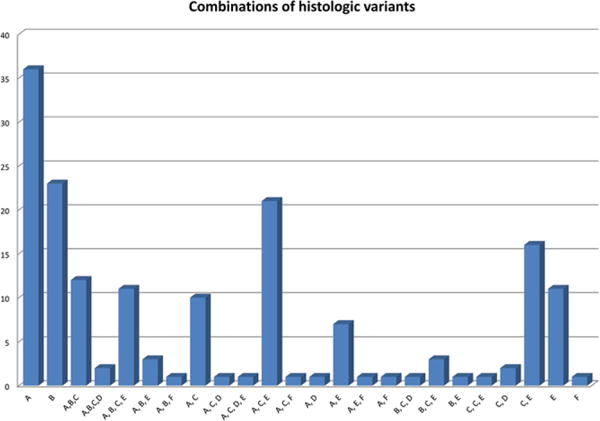
Each combination of histologic type seen in the study is represented, with those containing A, B, C, and E being most common; other combinations are seen one to three times
Our results concur with an emerging trend indicating variant histology in NLPHL to be more frequent in young people than in adults, the presence of LP cells in the extranodular T-cell areas (Fan type C) to be common in young patients, cytoplasmic IgD expression to be associated with types C and E variant histology. Dim or focal CD30 expression is not unusual and does not impart adverse risk in this setting. Variant histology type C with prominent extranodular large B cells involving > 25% of the sample showed an association with increased relapse. Type D, with T-cell-rich nodules, also showed increased relapse when >25% but the number of those patients (n = 3) was too small to draw conclusions. Our study suggests that delineation of variant histology and performance of IgD staining may be helpful to identify patients for whom increased surveillance may be indicated, however, the outcome of the study remains excellent. Overall survival is 100% and variant histology does not imply that limited therapy is contraindicated if clinical follow-up is adequate.
Acknowledgments
We thank Julie Lippa, Helene Degan, and Donna Barrett (SUNY Upstate Medical University) for assistance with immunohistochemical staining. We also thank the administrative staff, individual institutional pathologists, oncologists, and staff of the Children’s Oncology Group for submission of cases. Research reported in this publication was supported by the Children’s Oncology Group, National Cancer Institute of the National Institutes of Health under award numbers U10CA098543 and U10CA098413, NCTN Operations Center Grant U10CA180886, and NCTN Statistics & Data Center Grant U10CA180899. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- AV-PC
doxorubicin/vincristine/prednisone/cyclophosphamide
- CHL
classical Hodgkin lymphoma
- EBER
EBV-encoded ribonucleotide
- EBV
Epstein–Barr virus
- EFS
event-free survival
- HL
Hodgkin lymphoma
- IgD
Immunoglobulin D
- IHC
immunohistochemical
- LP
lymphocyte predominant
- NLPHL
nodular lymphocyte predominant Hodgkin lymphoma
Footnotes
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
References
- 1.Appel BE, Chen L, Buxton AB, et al. Minimal treatment of low-risk pediatric lymphocyte predominant Hodgkin lymphoma: a report from the Children’s Oncology Group. J Clin Oncol. 2016;34:2372–2379. doi: 10.1200/JCO.2015.65.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swerdlow SH, Campo E, Harris NL, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC; Lyon: 2008. [Google Scholar]
- 3.Anagnostopoulos I, Hansmann ML, Franssila K, et al. European Task Force on Lymphoma project on lymphocyte predominance Hodgkin disease: histologic and immunohistologic analysis of submitted cases reveals 2 types of Hodgkin disease with a nodular growth pattern and abundant lymphocytes. Blood. 2000;96(5):1889–1899. [PubMed] [Google Scholar]
- 4.Nicholas DS, Harris S, Wright DH. Lymphocyte predominance Hodgkin’s disease—an immunohistochemical study. Histopathology. 1990;16(2):157–165. doi: 10.1111/j.1365-2559.1990.tb01084.x. [DOI] [PubMed] [Google Scholar]
- 5.Poppema S, Delsol G, Pileri SA, et al. Nodular lymphocyte predominant Hodgkin lymphoma. In: Swerdlow SH, Campo E, Harris NL, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC; Lyon: 2008. pp. 323–325. [Google Scholar]
- 6.Fan Z, Natkunam Y, Bair E, Tibshirani R, Warnke RA. Characterization of variant patterns of nodular lymphocyte predominant Hodgkin lymphoma with immunohistologic and clinical correlation. Am J Surg Pathol. 2003;27(10):1346–1356. doi: 10.1097/00000478-200310000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Hartmann S, Eichenauer DA, Plütschow A, et al. Histopathological features and their prognostic impact in nodular lymphocyte-predominant Hodgkin lymphoma—a matched pair analysis from the German Hodgkin Study Group (GHSG) Br J Haematol. 2014;167(2):238–242. doi: 10.1111/bjh.12997. [DOI] [PubMed] [Google Scholar]
- 8.Fanale M. A novel prognostic scoring system for NLPHL. Blood. 2013;122(26):4154–4155. doi: 10.1182/blood-2013-11-533109. [DOI] [PubMed] [Google Scholar]
- 9.Shankar AG, Kirkwood AA, Hall GW, Hayward J, O’Hare P, Ramsay AD. Childhood and adolescent nodular lymphocyte predominant Hodgkin lymphoma—a review of clinical outcome based on the histological variants. Br J Haematol. 2015;171:254–262. doi: 10.1111/bjh.13540. [DOI] [PubMed] [Google Scholar]
- 10.Shet T, Panjwani P, Epari S, et al. A simplified M scoring system to document variant patterns in nodular lymphocyte predominant Hodgkin lymphoma. Leuk Lymph. 2015;56:1651–1658. doi: 10.3109/10428194.2014.961013. [DOI] [PubMed] [Google Scholar]
- 11.Ranjan P, Naresh KN. CD30 expression in L&H cells of Hodgkin’s disease, nodular lymphocyte predominant type. Histopathology. 2003;42(4):406–407. doi: 10.1046/j.1365-2559.2003.01552_3.x. [DOI] [PubMed] [Google Scholar]
- 12.Seliem RM, Ferry JA, Hasserjian RP, Narris NL, Zukerberg LR. Nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL) with CD30-positive lymphocyte-predominant (LP) cells. J Hematopathol. 2011;4:175–181. doi: 10.1007/s12308-011-0104-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huppmann AR, Nicolae A, Slack GW, et al. EBV may be expressed in the LP cells of nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL) in both children and adults. Am J Surg Pathol. 2014;38(3):316–324. doi: 10.1097/PAS.0000000000000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang S, Medeiros LJ, Xu-Monette ZY, et al. Epstein-Barr virus-positive nodular lymphocyte predominant Hodgkin lymphoma. Ann Diagn Pathol. 2014;18(4):203–209. doi: 10.1016/j.anndiagpath.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 15.O’Sullivan CC, Ozdemirli M, Bazylewicz M, Cheson BD. Complete response to brentuximab vedotin in a transplant-naïve patient with relapsed CD30-positive nodular lymphocyte-predominant Hodgkin lymphoma. Clin Adv Hematol Oncol. 2013;11(6):382–385. [PubMed] [Google Scholar]
- 16.Prakash S, Fountaine T, Raffeld M, Jaffe ES, Pittaluga S. IgD positive L&H cells identify a unique subset of nodular lymphocyte predominant Hodgkin lymphoma. Am J Surg Pathol. 2006;30(5):585–592. doi: 10.1097/01.pas.0000194741.87798.45. [DOI] [PubMed] [Google Scholar]
- 17.Karayalcin G, Behm FG, Gieser PW, et al. Lymphocyte predominant Hodgkin disease: clinico-pathologic features and results of treatment—the Pediatric Oncology Group experience. Med Pediatr Oncol. 1997;29(6):519–525. doi: 10.1002/(sici)1096-911x(199712)29:6<519::aid-mpo1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 18.Fanale M. Lymphocyte-predominant Hodgkin lymphoma: what is the optimal treatment? Hematol Am Soc Hematol Educ Program. 2013;2013:406–413. doi: 10.1182/asheducation-2013.1.406. [DOI] [PubMed] [Google Scholar]
- 19.Hartmann S, Eichenauer DA, Plütschow A, et al. The prognostic impact of variant histology in nodular lymphocyte-predominant Hodgkin lymphoma: a report from the German Hodgkin Study Group (GHSG) Blood. 2013;122(26):4246–4242. doi: 10.1182/blood-2013-07-515825. [DOI] [PubMed] [Google Scholar]
- 20.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xing KH, Connors JM, Lai A, et al. Advanced-stage nodular lymphocyte predominant Hodgkin lymphoma compared with classical Hodgkin lymphoma: a matched pair outcome analysis. Blood. 2014;123(23):3567–3573. doi: 10.1182/blood-2013-12-541078. [DOI] [PubMed] [Google Scholar]
- 22.Wilder RB, Schlembach PJ, Jones D, et al. European Organization for Research and Treatment of Cancer and Groupe d’Etude des Lymphomes de l’Adulte very favorable and favorable, lymphocyte-predominant Hodgkin disease. Cancer. 2002;94(6):1731–1738. doi: 10.1002/cncr.10404. [DOI] [PubMed] [Google Scholar]


