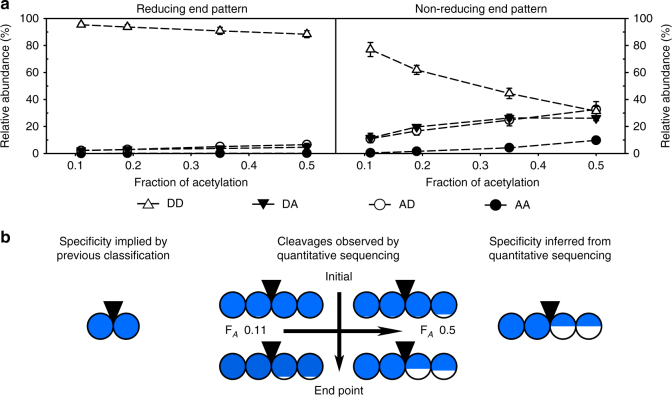
Fig. 4.
Specificity of CSN-MN (being highly similar to CSN-7M representing class II) inferred from its products. (a) The patterns of acetylation of the two terminal sugar units were analyzed for different FA of the substrates by quantitative sequencing of the products at the endpoint of enzymatic hydrolysis. The molar fractions (mean values of at least three independent measurements of three independent enzyme batches) of all possible diads (DD, DA, AD, and AA) are shown. (b) The specificity of class II chitosanases implicated by the established qualitative classification system compared to the specificity of CSN-MN (97% similar to the model class II chitosanase CSN-7M) inferred from the cleavages observed by the quantitative sequencing of its products’ sugar moieties. Diad frequencies at the early time points of enzymatic hydrolysis are taken from Supplementary Table 5. Detailed oligomer compositions are shown in Supplementary Table 3. The circles are divided according to the percentage of the relative abundances (molar fractions) of GlcN (blue) and GlcNAc (white) at the two corresponding subsites, left, (−2) and (−1), and right, (+1) and (+2), of the catalytic cleavage site (indicated by a black inverted triangle). The mean values with standard deviations of at least three independent measurements of three independent enzyme batches are shown (Supplementary Tables 3 and 5)