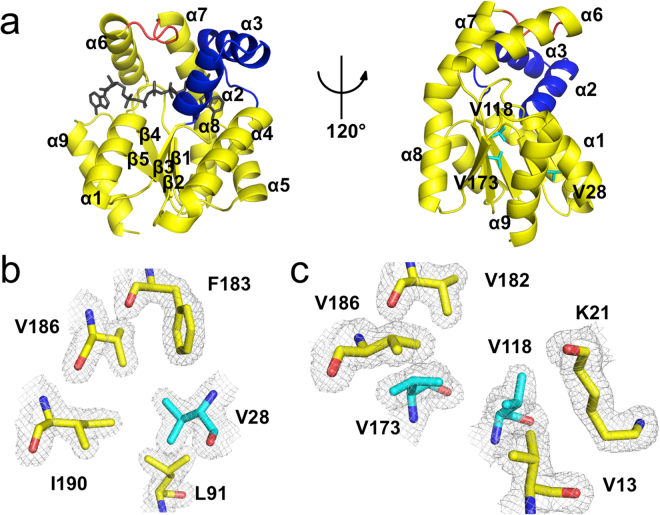
Figure 2.
Crystal structure of the Antarctic AKNc. (a) Overall structure of AKNc. The CORE (residues 1–38, 69–136, and 143–193), AMPbind (residues 39–68) and LID (residues 137–142) domains are shown in yellow, blue, and red, respectively. The co-crystallized Ap5A molecule bound to the active site is shown in black (left), but not in the 120°-rotated model (right), to reveal the AK structure more clearly. The three Val residues for which hydrophobic contacts are suboptimal are highlighted in cyan in stick representations (right). (b,c) Close-up views of the hydrophobic environment around Val28 (b) and Val118/Val173 (c). The Val residues interact hydrophobically with other residues in the CORE domain, but there is room for improvement in hydrophobic packing. The 2mFobs − DFcalc map is contoured at 1.0 σ.