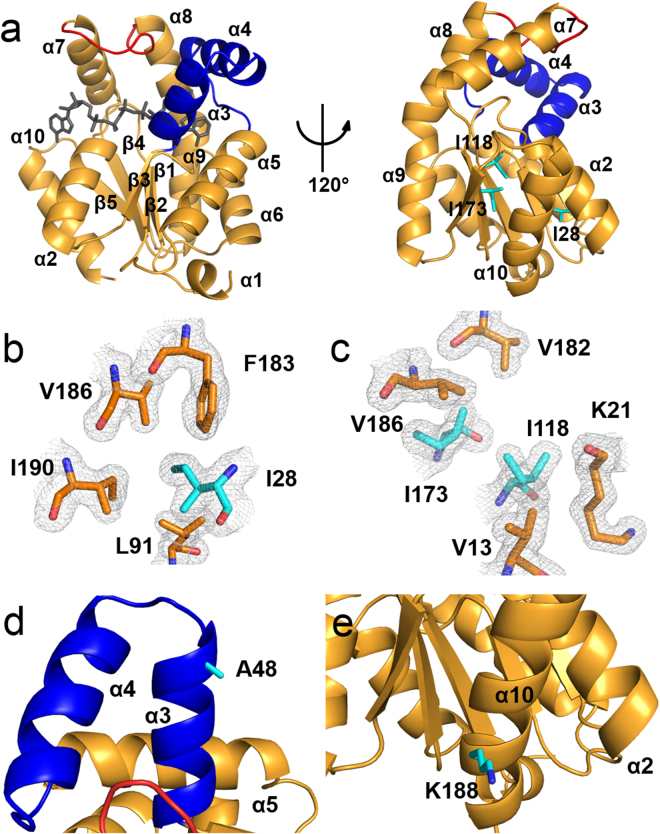
Figure 3.
Crystal structure of the tropical AKDr. (a) Overall structure of AKDr. The CORE (residues 1–38, 69–136, and 143–194), AMPbind (residues 39–68) and LID (residues 137–142) domains are shown in orange, blue, and red, respectively. The co-crystallized Ap5A molecule is shown in black. (b,c) Hydrophobic contacts of Ile28 (b) and Ile118/Ile173 (c) in the CORE domain of the AKDr structure. The three Ile residues are substituted to Val residues in AKNc. The 2mFobs−DFcalc map is contoured at 1.0 σ. (d,e) Close-up views of Ala48 (d) and Lys188 (e) in the crystal structure of AKDr. Ala48 and Lys188 are conserved among the three tropical AKs including AKDr, but not in AKNc. The side chains of Ala48 and Lys188 are exposed to the solvent, and do not interact closely with residues that are located distantly in the amino acid sequence.