Figure 4.
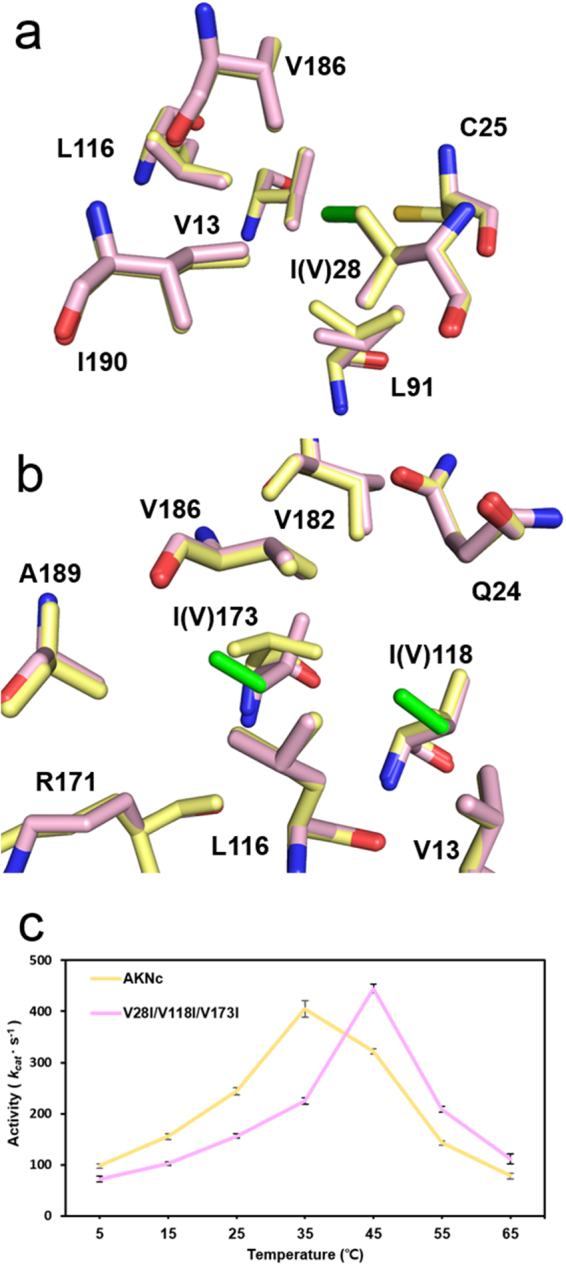
Optimization of hydrophobic CORE packing by Val-to-Ile substitutions in AKNc. (a,b) V28I (a) and V118I/V173I (b) substitutions improve CORE packing with other hydrophobic residues in the crystal structure of the V28I/V118I/V173I mutant of AKNc (pink). The three mutated Ile residues are highlighted in green. The structure of WT AKNc (yellow) is aligned with that of the mutant. (c) Temperature dependence of activity of WT AKNc and the V28I/V118I/V173I mutant. The activities in the direction of ATP formation were measured at various temperatures. The Val-to-Ile mutations reduced the catalytic activity at low temperatures (5–35 °C). At each temperature, three independent measurements were made. Data are represented as mean ± standard error of mean.
