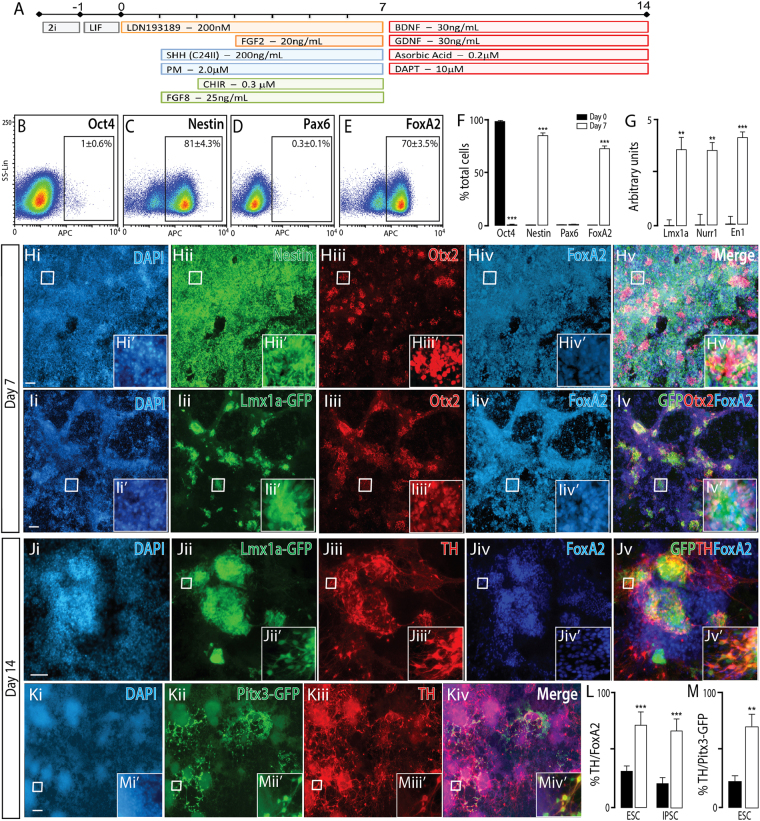
Figure 6.
Naïve mESC differentiated to a ventral midbrain fate, show appropriate regional and temporal specification. (A) Schematic of the neural differentiation protocol, detailing morphogen and small molecules employed, to generate ventral midbrain progenitors and neurons from naïve PSC. (B–E) Flow cytometry plots at day 7 and, (F) quantitative analysis at day 0 and 7 for Oct4, Nestin, Pax6 and FoxA2, showing appropriate downregulaton of pluripotent (Oct4) and dorsal (Pax6) markers together with upregulation of neural (Nestin) and ventral (FoxA2) expression. (G) Transcriptional expression of ventral midbrain genes Lmx1a, Nurr1 and En1. (H) Representative photomicrographs depicting expression of Nestin, Otx2 and FoxA2 in VM progenitors at day7. (I) Employment of an Lmx1a-GFP reporter line demonstrated high co-expression with Otx2 and FoxA2, indicative of ventral mesencephalic progenitors. (J) By day 14, these progenitors matured into VM dopaminergic neurons co-expressing the hallmark proteins Lmx1a, TH and FoxA2. (K) Representative images depicting Pitx3-GFP and TH expression within mature cultures, confirming ventral midbrain dopaminergic fate. (L) Quantification of %TH/FoxA2 neurons from VM (white) and ventral forebrain (black bars) cultures. Elevated proportions of TH+ FoxA2+ co-expressing neurons confirmed a VM dopaminergic phenotype, while FoxA2+ TH+ cells within VF cultures were indicative of off-target populations. (M) Quantification of TH+ /Pitx3-GFP+ neurons within ventral forebrain and ventral midbrain cultures at day 14, using the Pitx3-GFP reporter mESC line, confirmed the generation of bona fide VM dopaminergic neurons within VM cultures. Data represents mean ± SEM, n = 3, Students t-test. Scale bar = 100 um. **p < 0.01, ***p < 0.001.