Abstract
Kainate receptors require the presence of external ions for gating. Most work thus far has been performed on homomeric GluK2 but, in vivo, kainate receptors are likely heterotetramers. Agonists bind to the ligand-binding domain (LBD) which is arranged as a dimer of dimers as exemplified in homomeric structures, but no high-resolution structure currently exists of heteromeric kainate receptors. In a full-length heterotetramer, the LBDs could potentially be arranged either as a GluK2 homomer alongside a GluK5 homomer or as two GluK2/K5 heterodimers. We have constructed models of the LBD dimers based on the GluK2 LBD crystal structures and investigated their stability with molecular dynamics simulations. We have then used the models to make predictions about the functional behavior of the full-length GluK2/K5 receptor, which we confirmed via electrophysiological recordings. A key prediction and observation is that lithium ions bind to the dimer interface of GluK2/K5 heteromers and slow their desensitization.
Main Text
GluK2 and GluK5 are the most widely expressed kainate receptor subunits in the central nervous system (CNS) (1, 2). In coexpressing cells, they form GluK2/K5 heterotetramers with a 2:2 stoichiometry (3), exhibiting distinct functional and pharmacological properties, which differ significantly from the more studied GluK2 homotetramer (2, 4). For example, the presence of the GluK5 subunit in the receptor increases the sensitivity to agonists such as glutamate (5) or kainate (2), and provides an increased response to α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) (2). Thus, despite belonging to the same family, GluK5 possesses unique features that help to broaden the functional and pharmacological profile of kainate receptors, which have been identified as a potential target for the treatment of mood disorders, epilepsy, and pain perception (6). Indeed, GluK2/K5 receptors play a major role in chronic and recurrent seizures in temporal lobe epilepsy (7).
Despite their inability to form functional homomeric channels, the secondary subunits, GluK4 and GluK5, are important at synapses and the ablation of the genes encoding these subunits in mice leads to the complete suppression of the synaptic kainate receptor-mediated currents (8). This suggests that heteromeric complexes including these two subunits are essential for the ionotropic function of the kainate receptors in vivo, and thus an atomically detailed model of the heteromer, particularly at the level of the ligand-binding domains (LBDs), would be very useful in furthering our understanding of these receptors. Unfortunately, despite the in vivo relevance of the GluK2/K5 heterotetramer (8), experimental difficulties in dealing with heterogeneity have hindered the determination of its structural arrangement so far. Given the high structural similarity among ionotropic glutamate receptors (iGluRs), one would expect a GluK2/K5 heteromer to share the commonly observed symmetry mismatch between the extracellular domain, which arranges as a dimer of dimers, and the fourfold symmetric transmembrane domain (9, 10, 11, 12, 13). Despite such overall structural similarity, there is growing evidence that minor changes in composition can have dramatic influences on function, and therefore any similarities should be extrapolated with caution. In particular, a series of point mutations at the dimer interface of the LBDs have been shown to alter the receptor kinetics by either delaying the onset of desensitization (14, 15) or even disrupting the gating of the receptor (16).
However, functional effects provide an opportunity to validate a potential model of GluK2/K5. Indeed, as with any model, the usefulness can be assessed by its ability to make functional predictions. Here, we present an atomistic model of the LBD dimer of the physiologically relevant GluK2/K5 receptor. The LBDs of iGluRs are useful as a reduced model of receptor gating, as they have been shown to reproduce ligand-binding affinities (17) as well as conserving similar structural features present when part of the full-length receptor (18). Moreover, changes in the interfacial interactions within LBD dimers (18, 19) are believed to drive the receptor state transitions. Additionally, the presence of three monovalent modulatory ions at the LBD interface are key for the stability of the conducting configuration of kainate receptors (20, 21). Furthermore, the release of two of these ions from the LBD marks the start of the desensitization process (16) through the separation of the LBD interface (9, 11).
In this study, we show how initial structural stability assessment of potential LBD combinations suggests that the LBDs are most likely comprised of GluK2/K5 heterodimers and that this model can predict an effect of Li+ on the desensitization properties of the full-length receptor. The effects of Li+ were confirmed by electrophysiological recordings. The foremost uncertainty regarding the structure of the GluK2/K5 heteromers is the LBD composition itself. Although some experimental data suggest that the heterotetrameric receptor might be composed of a dimer of GluK2/K5 heterodimers (22, 23), the possibility of a LBD formed by two GluK2 and GluK5 homodimers has not yet been ruled out. Therefore, we generated and assessed models of GluK2/K5 and GluK5/K5 LBDs. Simulations were also compared to simulations of the experimentally resolved GluK2 homodimer structure (19) (see the Supporting Material).
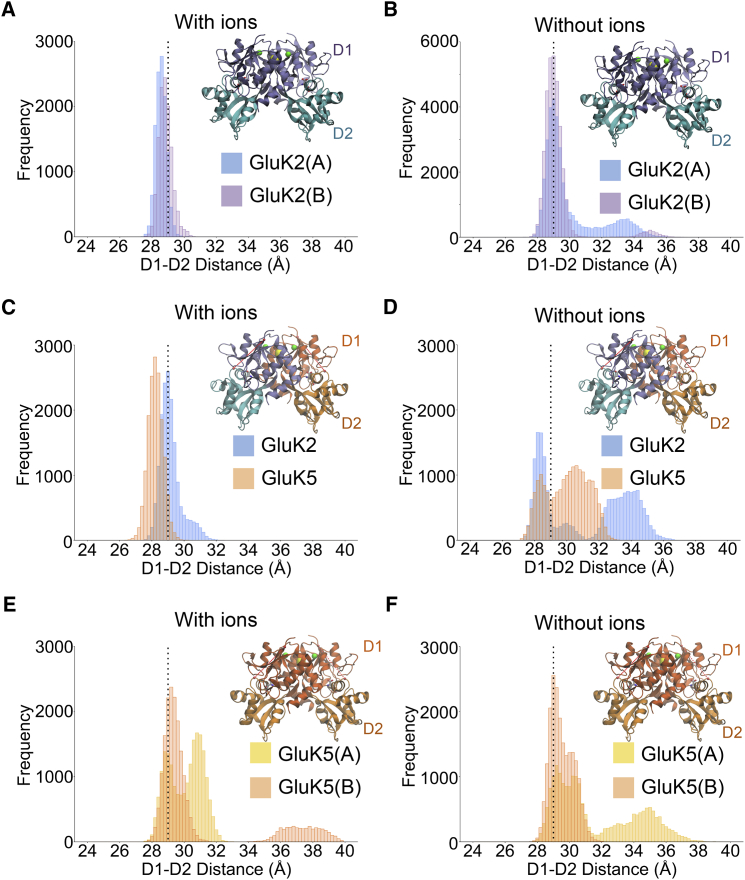
As an initial assessment of structural stability, we monitored the behavior of the individual LBD lobes (D1 and D2) in maintaining a closed-cleft conformation in the presence of glutamate. The D1-D2 distance of the LBD domains has been previously used as a structural marker of iGluR amplitude response (24, 25, 26), although we note that it may not linearly correspond to response amplitude (27). It is generally thought that the presence of partial agonists correlates with a lower degree of D1-D2 closure, which could in turn decrease the frequency at which the receptors access the highest conductance states (28). As kainate receptors are known to require ions for function, we performed molecular dynamics simulations with and without ions (Na+ and Cl−) present. Dimers of GluK2 and GluK2/K5 maintain a gating configuration when the modulatory ions are bound, and move away from that state when the ions are absent (Fig. 1), reflecting loss of stability in terms of cleft closure.
Figure 1.
D1-D2 distance distributions from the last 20 ns of five independent simulations for the GluK2 homomer (A and B), the GluK2/K5 heteromer (C and D), and the GluK5 homomer (E and F) (additional data for B was available for GluK2 without ions from previous simulations (16, 34)) in the presence and absence of modulatory sodium (green spheres) and chloride (yellow spheres) ions. The frequency distributions use 0.2 Å bins. Simulations with ions were manually verified and were omitted from the “with ion” distributions if there was dissociation. The dashed vertical line indicates the equivalent distance in the GluK2 homodimer crystal structure (3G3F) (19). To see this figure in color, go online.
Interestingly, the GluK5 homodimer was unstable both with and without ions, and more likely to exist as an open-cleft conformation (Fig. 1). Although the link between the number of closed clefts and subconductance states is established for AMPA receptors but not for kainate receptors (29), these results tend to support the hypothesis that GluK2/K5 receptors are more likely formed by a dimer of GluK2/K5 heterodimers (4, 23, 30).
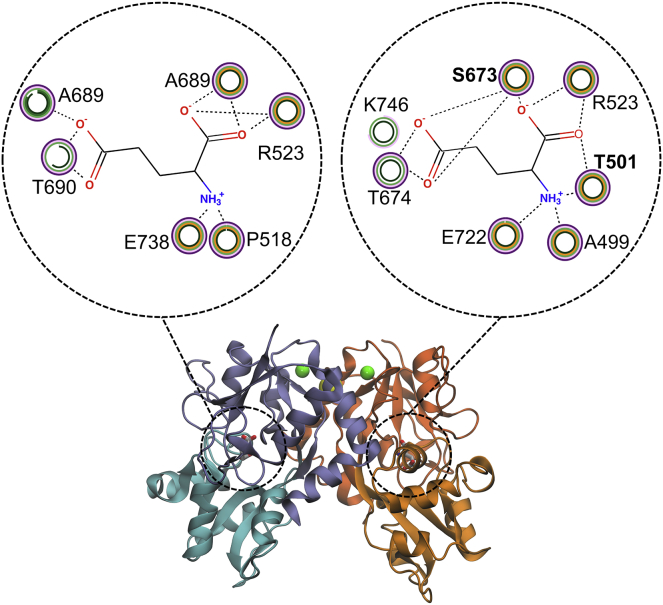
The relevance of ion binding in the gating configuration of the GluK2 homodimer and GluK2/K5 heterodimer became evident in two of the “bound” simulations, where the cations spontaneously unbind from the LBD interface (Fig. S1). In both systems, the release of one of the two cations was followed by the opening of the clamshell of GluK2, an event not observed in any of the simulations in which Na+ ions remained bound. Of note, the key “anchor” residue, R523, in the binding site (Figs. 2 and S3) is adjacent to E524, which interacts directly with one of the cations, thereby providing a direct link between the dynamics of the interface and the behavior of the binding site (and consequently the stability of the clamshell). Interestingly, several additional hydrogen bonds are formed between glutamate and GluK5, facilitated by the highly conserved (between species) residues T501 and S673 (bold in Fig. 2), which are both alanines in GluK2. The higher number of interactions is consistent with the higher binding affinity of GluK5 for glutamate.
Figure 2.
Glutamate binding interactions (from the last 20 ns of five repeat simulations) observed in GluK2 and GluK5 within the GluK2/K5 heterodimer, where the dashed line represents a hydrogen bond and the five circular rings indicate the frequency of these interaction within each of the five simulation repeats (no color indicates no interaction in that repeat). S673 and T501 (bold) contribute additional interactions from the GluK5 subunit. To see this figure in color, go online.
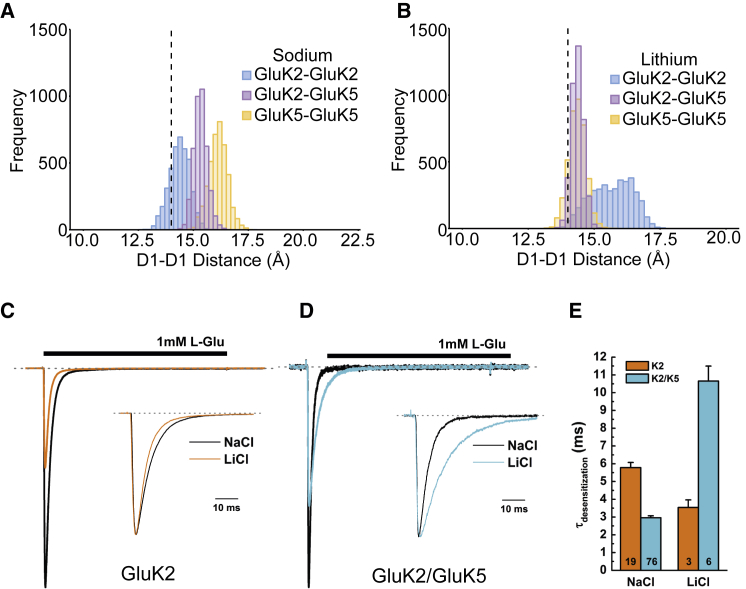
Given the requirement of the Na+ ions for dimer stability, we tested the effect of Li+ ions on the structural properties of the LBDs following our previous approach for GluK2 homomers (31) and similar to our recent approach to examining desensitization kinetics in AMPA receptors (32). Here, we repeated the simulations of the three LBDs studied, replacing Na+ with Li+ in the salt buffer (Fig. 3, A and B). In our simulations, Li+ ions remained bound to the LBD. Given the previously documented role of ions at this interface in the process of desensitization, we examined the D1-D1 distances at the dimer interface, between subunits (see Fig. S2 for definitions) for each of the systems (Fig. 3). Several things are noteworthy. Firstly, in the presence of sodium, the D1-D1 equilibrium distance (peak of the histogram) shifts depending on the nature of the dimer composition (Fig. 3 A).
Figure 3.
Sodium stabilizes GluK2 homomer models (A) but lithium does not (B). Lithium does, however, stabilize GluK2/K5 heteromer models. Recordings in the presence of different cations for the GluK2 homomer and GluK2/K5 heteromer show different responses (C and D). Example electrophysiological traces showing responses in NaCl (black) and LiCl (orange/cyan) background (GluK2 (C), GluK2/K5 (D)). Insets show peak-normalized responses to highlight changes in decay kinetics. (E) Analysis of the fast exponential decay component (τfast) for GluK2 and GluK2/K5 in NaCl and LiCl background. Bars indicate the mean and error bars are mean ± SE; the number of patches is shown on each bar. To see this figure in color, go online.
Specifically, the GluK2/K5 distance is increased (to 15.4 Å) and the GluK5/K5 distance is even further increased to 16.2 Å (Fig. 3 A). In the presence of Li+, (Fig. 3 B), the GluK2/K2 distribution is broader and centered at a higher separation when compared to Na+. In contrast, the separations for the GluK2/K5 and GluK5/K5 dimers are reduced compared to the distances observed in the presence of Na+; the values were in fact similar to those observed in the GluK2/K2 crystal structure (19). Since the distances here have previously been linked to receptor desensitization in GluK2 homomers (16), this suggested to us that the presence of Li+ in GluK5-containing receptors should result in slower desensitization. To test this, we studied the rates of desensitization for recombinantly-expressed GluK2 and GluK2/K5 using outside-out patches (see Supporting Material). As previously shown (33), the decay kinetics of GluK2 responses were accelerated in the presence of LiCl (Fig. 3 C). Interestingly, GluK2/K5 decay kinetics were slowed by more than threefold in LiCl (Fig. 3, D and E). These results are in agreement with the simulation data and suggest that, similar to AMPA receptors, the stability of the LBD dimer interface of GluK2/GluK5 regulates desensitization kinetics (Fig. 3, C–E). It will be interesting to see the effects, if any, of other cations on GluK5.
To ascertain the thermodynamic impact of Li+ on the LBD of the heterotetramer, we calculated the free energy of binding (affinity) of Na+ and Li+ in both the GluK2 homodimer and the GluK2/K5 heterodimer using free energy perturbation, as previously described (31). The results showed that although both systems preferably bind to Li+, a ∼3 kcal/mol higher affinity is observed in the heterodimer (see Table S1 for details). Such a difference suggests that the tighter packing of the D1-D1 segments of the GluK2/K5 LBD in the presence of Li+ (Fig. 3 B) could result in a higher energy barrier to be overcome during the D1-D1 separation movements that are believed to trigger desensitization. The mechanism with which Li+ ions exert their effect on GluK2/K5 heteromers appears to result from tighter interface packing compared to when Na+ ions are present. Indeed, the solvent density across the different systems is smaller in the presence of Li+ (Fig. S3). In GluK5-containing models, there is a smaller volume of water density accessible to the ions and the Li+ appears to mediate more cross-dimer interactions. The distribution of direct water-cation interactions also supports this (Fig. S4). These observations are in agreement with our previous studies examining the dynamic behavior of this interface (16, 32, 34).
Author Contributions
D.B. and P.C.B. designed the research. T.P., M.M., and P.M.G.E.B. performed the research and analyzed the results. T.P. and P.C.B. wrote the article.
Acknowledgments
We thank L. Domicevica, G. Gerogiokas, and M.R.P. Aurousseau for discussions.
T.P. was funded by the Medical Research Council (MRC) (MR/M000435/1), M.M. by the Alfred Benzon Foundation, P.M.G.E.B. by the Fonds de Recherche en Santé du Québec, and D.B. by the Canadian Institutes of Health Research. We thank the Advanced Research Computing facility, the Engineering and Physical Sciences Research Council (EPSRC) National Service for Computational Chemistry Software (NSCCS) (EP/J003921/1), and the ARCHER UK National Supercomputing Services for computer time granted via the High-End Computing Consortium for Biomolecular Simulation (http://www.hecbiosim.ac.uk), supported by the EPSRC (EP/L000253/1). Work in the P.C.B. laboratory is supported by the Biotechnology and Biological Sciences Research Council and the MRC.
Editor: Vasanthi Jayaraman.
Footnotes
Supporting Materials and Methods, four figures, and one table are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(17)30970-0.
Supporting Material
References
- 1.Petralia R.S., Wang Y.-X., Wenthold R.J. Histological and ultrastructural localization of the kainate receptor subunits, KA2 and GluR6/7, in the rat nervous system using selective antipeptide antibodies. J. Comp. Neurol. 1994;349:85–110. doi: 10.1002/cne.903490107. [DOI] [PubMed] [Google Scholar]
- 2.Herb A., Burnashev N., Seeburg P.H. The KA-2 subunit of excitatory amino acid receptors shows widespread expression in brain and forms ion channels with distantly related subunits. Neuron. 1992;8:775–785. doi: 10.1016/0896-6273(92)90098-x. [DOI] [PubMed] [Google Scholar]
- 3.Reiner A., Arant R.J., Isacoff E.Y. Assembly stoichiometry of the GluK2/GluK5 kainate receptor complex. Cell Reports. 2012;1:234–240. doi: 10.1016/j.celrep.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher J.L., Mott D.D. Distinct functional roles of subunits within the heteromeric kainate receptor. J. Neurosci. 2011;31:17113–17122. doi: 10.1523/JNEUROSCI.3685-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barberis A., Sachidhanandam S., Mulle C. GluR6/KA2 kainate receptors mediate slow-deactivating currents. J. Neurosci. 2008;28:6402–6406. doi: 10.1523/JNEUROSCI.1204-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lerma J., Marques J.M. Kainate receptors in health and disease. Neuron. 2013;80:292–311. doi: 10.1016/j.neuron.2013.09.045. [DOI] [PubMed] [Google Scholar]
- 7.Crépel V., Mulle C. Physiopathology of kainate receptors in epilepsy. Curr. Opin. Pharmacol. 2015;20:83–88. doi: 10.1016/j.coph.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Fernandes H.B., Catches J.S., Contractor A. High-affinity kainate receptor subunits are necessary for ionotropic but not metabotropic signaling. Neuron. 2009;63:818–829. doi: 10.1016/j.neuron.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyerson J.R., Kumar J., Subramaniam S. Structural mechanism of glutamate receptor activation and desensitization. Nature. 2014;514:328–334. doi: 10.1038/nature13603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karakas E., Furukawa H. Crystal structure of a heterotetrameric NMDA receptor ion channel. Science. 2014;344:992–997. doi: 10.1126/science.1251915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dürr K.L., Chen L., Gouaux E. Structure and dynamics of AMPA receptor GluA2 in resting, pre-open, and desensitized states. Cell. 2014;158:778–792. doi: 10.1016/j.cell.2014.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sobolevsky A.I., Rosconi M.P., Gouaux E. X-ray structure, symmetry and mechanism of an AMPA-subtype glutamate receptor. Nature. 2009;462:745–756. doi: 10.1038/nature08624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee C.H., Lü W., Gouaux E. NMDA receptor structures reveal subunit arrangement and pore architecture. Nature. 2014;511:191–197. doi: 10.1038/nature13548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y., Nayeem N., Green T. Interface interactions modulating desensitization of the kainate-selective ionotropic glutamate receptor subunit GluR6. J. Neurosci. 2006;26:10033–10042. doi: 10.1523/JNEUROSCI.2750-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nayeem N., Mayans O., Green T. Correlating efficacy and desensitization with GluK2 ligand-binding domain movements. Open Biol. 2013;3:130051. doi: 10.1098/rsob.130051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dawe G.B., Musgaard M., Bowie D. Defining the structural relationship between kainate-receptor deactivation and desensitization. Nat. Struct. Mol. Biol. 2013;20:1054–1061. doi: 10.1038/nsmb.2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuusinen A., Arvola M., Keinänen K. Molecular dissection of the agonist binding site of an AMPA receptor. EMBO J. 1995;14:6327–6332. doi: 10.1002/j.1460-2075.1995.tb00323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Traynelis S.F., Wollmuth L.P., Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol. Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaudhry C., Weston M.C., Mayer M.L. Stability of ligand-binding domain dimer assembly controls kainate receptor desensitization. EMBO J. 2009;28:1518–1530. doi: 10.1038/emboj.2009.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plested A.J.R., Vijayan R., Mayer M.L. Molecular basis of kainate receptor modulation by sodium. Neuron. 2008;58:720–735. doi: 10.1016/j.neuron.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bowie D. External anions and cations distinguish between AMPA and kainate receptor gating mechanisms. J. Physiol. 2002;539:725–733. doi: 10.1113/jphysiol.2001.013407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar J., Schuck P., Mayer M.L. Structure and assembly mechanism for heteromeric kainate receptors. Neuron. 2011;71:319–331. doi: 10.1016/j.neuron.2011.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reiner A., Isacoff E.Y. Tethered ligands reveal glutamate receptor desensitization depends on subunit occupancy. Nat. Chem. Biol. 2014;10:273–280. doi: 10.1038/nchembio.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krintel C., Frydenvang K., Kastrup J.S. L-Asp is a useful tool in the purification of the ionotropic glutamate receptor A2 ligand-binding domain. FEBS J. 2014;281:2422–2430. doi: 10.1111/febs.12795. [DOI] [PubMed] [Google Scholar]
- 25.Lau A.Y., Roux B. The hidden energetics of ligand binding and activation in a glutamate receptor. Nat. Struct. Mol. Biol. 2011;18:283–287. doi: 10.1038/nsmb.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armstrong N., Gouaux E. Mechanisms for activation and antagonism of an AMPA-sensitive glutamate receptor: crystal structures of the GluR2 ligand binding core. Neuron. 2000;28:165–181. doi: 10.1016/s0896-6273(00)00094-5. [DOI] [PubMed] [Google Scholar]
- 27.Fay A.-M.L., Corbeil C.R., Bowie D. Functional characterization and in silico docking of full and partial GluK2 kainate receptor agonists. Mol. Pharmacol. 2009;75:1096–1107. doi: 10.1124/mol.108.054254. [DOI] [PubMed] [Google Scholar]
- 28.Jin R., Banke T.G., Gouaux E. Structural basis for partial agonist action at ionotropic glutamate receptors. Nat. Neurosci. 2003;6:803–810. doi: 10.1038/nn1091. [DOI] [PubMed] [Google Scholar]
- 29.Smith T.C., Howe J.R. Concentration-dependent substrate behavior of native AMPA receptors. Nat. Neurosci. 2000;3:992–997. doi: 10.1038/79931. [DOI] [PubMed] [Google Scholar]
- 30.Fisher J.L. The neurotoxin domoate causes long-lasting inhibition of the kainate receptor GluK5 subunit. Neuropharmacology. 2014;85:9–17. doi: 10.1016/j.neuropharm.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vijayan R., Plested A.J.R., Biggin P.C. Selectivity and cooperativity of modulatory ions in a neurotransmitter receptor. Biophys. J. 2009;96:1751–1760. doi: 10.1016/j.bpj.2008.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dawe G.B., Musgaard M., Bowie D. Distinct structural pathways coordinate the activation of AMPA receptor-auxiliary subunit complexes. Neuron. 2016;89:1264–1276. doi: 10.1016/j.neuron.2016.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong A.Y.C., Fay A.-M.L., Bowie D. External ions are coactivators of kainate receptors. J. Neurosci. 2006;26:5750–5755. doi: 10.1523/JNEUROSCI.0301-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Musgaard M., Biggin P.C. Steered molecular dynamics simulations predict conformational stability of glutamate receptors. J. Chem. Inf. Model. 2016;56:1787–1797. doi: 10.1021/acs.jcim.6b00297. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.