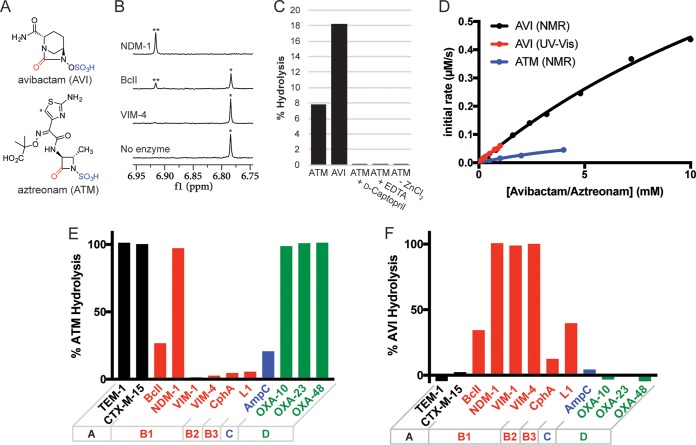
FIG 1.
Hydrolysis of avibactam and aztreonam by NDM-1. (A) Structures of avibactam and aztreonam. (B) Extent of aztreonam (100 μM) hydrolysis by VIM-4, BcII, and NDM-1 (all 10 μM) after 16 h. Hydrolysis was monitored by NMR spectroscopy (700 MHz); the peaks labeled with an asterisk correspond to the aztreonam proton indicated with an asterisk in panel A, while the peaks labeled with two asterisks indicate the hydrolyzed product. (C) Extent of hydrolysis of a mixture of aztreonam (ATM; 1 mM) and avibactam (AVI; 1 mM) by NDM-1 (10 μM) after 50 min with 100 μM ZnCl2–50 mM Tris-d11 (pH 7.5)–10% D2O. The addition of d-captopril (500 μM) or EDTA (2 mM) or removal of ZnCl2 inhibited aztreonam hydrolysis. (D) Preliminary kinetic characterization of avibactam and aztreonam hydrolysis by NDM-1 as monitored by NMR and UV-Vis analysis. The NMR studies used 10 μM NDM-1, 100 μM ZnCl2, and the indicated concentration of avibactam or aztreonam in 50 mM Tris-d11 (pH 7.5)–10% D2O. The UV-Vis studies (monitored at 230 nm) used 10 μM NDM-1, 20 μM ZnCl2, and the indicated concentration of avibactam in 5 mM HEPES, pH 7.5 (2). Nonlinear regression analyses were performed using Prism 7 (GraphPad). (E and F) Hydrolysis of (E) aztreonam and (F) avibactam by a panel of β-lactamases covering classes A (black), B (red), C (blue), and D (green). Hydrolysis was measured by NMR after 24 h for samples consisting of enzyme (10 μM) and avibactam (400 μM) or aztreonam (1 mM) in 50 mM Tris-d11 (pH 7.5)–10% D2O.