Abstract
Wasting embraces muscle and tissue wasting in sarcopenia and cachexia. This article describes recent advances in the field published in the Journal of Cachexia, Sarcopenia and Muscle concerning diagnostic tools, biomarker development, pathophysiology, and treatment. Studies discussed herein embrace those on sarcopenia and cachexia in heart failure, chronic obstructive pulmonary disease, and cancer including also animal models.
Keywords: Cachexia, Sarcopenia, Wasting, Heart failure, COPD, Cancer
Introduction
Wasting is an umbrella term that describes loss of lean and non‐lean tissues as well as loss in bone mineral density. Wasting thus embraces both sarcopenia and cachexia. From a clinical standpoint, sarcopenia may be divided into primary sarcopenia as part of the ageing process and into secondary sarcopenia that develops in the context of chronic illness. The term describes the loss skeletal muscle mass, which is not usually associated with weight loss but with loss of functioning muscle tissue and strength.1 Cachexia, on the other hand, develops as a consequence of a chronic inflammatory disease such as chronic heart failure, chronic kidney disease, chronic obstructive pulmonary disease (COPD), rheumatoid arthritis, or cancer. The determining feature of cachexia is the loss of body weight, which can be the result of loss in fat tissue, skeletal muscle, or both. Whenever such wasting is present in the context of chronic disease, this can usually be regarded as a signum mali ominis. Frailty, on the other hand, is a functional term that describes a functional result of muscle loss and cachexia leading to dependency, increased morbidity, and mortality, all of which are the result of functional decline leading, for example, to falls.2
The aim of the following overview is to provide insight into recent developments in the fields of body wasting and frailty with a focus on data published in the Journal of Cachexia, Sarcopenia and Muscle. The journal, founded in 2010, is focused on the dissemination of knowledge to both clinicians and researchers with a primary aim of highlighting data in the field of wasting across all the life sciences. Another important medium for dissemination is the now annual Cachexia Conference that was last held in Paris in 20153 and in Berlin in 2016, after which the Cachexia Conference went annual.4 Being such a niche journal, the journal now receives an increasing number of submissions, downloads as well as an increasing impact factor.5, 6 In this sense, the American architect, designer, and writer Richard Buckminster Fuller (1895–1983) was right when he wrote that ‘You never change things by fighting the existing reality. To change something, build a new model that makes the existing model obsolete’.
Sarcopenia
Prevalence, screening, and diagnosis
The diagnosis of sarcopenia remains rare outside the field of geriatrics, and therefore, the introduction of an ICD code for sarcopenia represents a major step forward in recognizing sarcopenia as a disease.7 Even though a number of bodies have recommended diagnostic criteria for sarcopenia, screening, use of diagnostic tools, and treatment remain underutilized.8 The heterogeneity of definitions proposed for the diagnosis of sarcopenia makes matters rather worse.9, 10, 11, 12, 13 Naturally, this fact is most important in the elderly. Using data from 3025 non‐disabled women aged 75 or older, the cross‐sectional EPIDOS study by Dupuy et al.14 showed the prevalence of sarcopenia ranging from 3.3% to 20.0% according to which one of six definitions was used (Figure 1). In this context, it was interesting to note that only 85 participants (3.1%) were identified to have sarcopenia according to all definitions.14 This study underscores the importance of having unified criteria that identify a clinically relevant cohort of affected subjects. Before such unifying definition is available and to better understand patients at risk of or with manifest sarcopenia, rapid screening remains of utmost importance. Morley and Cao15 have recently noted that the different definitions all embrace muscle function and muscle mass. However, they also acknowledge that muscle quality and therefore muscle performance are not directly related to muscle mass. This point is important because sarcopenia may also be a neuromuscular junction disease, and infiltration of fat into muscle during the ageing process precludes the development of weight loss.
Figure 1.
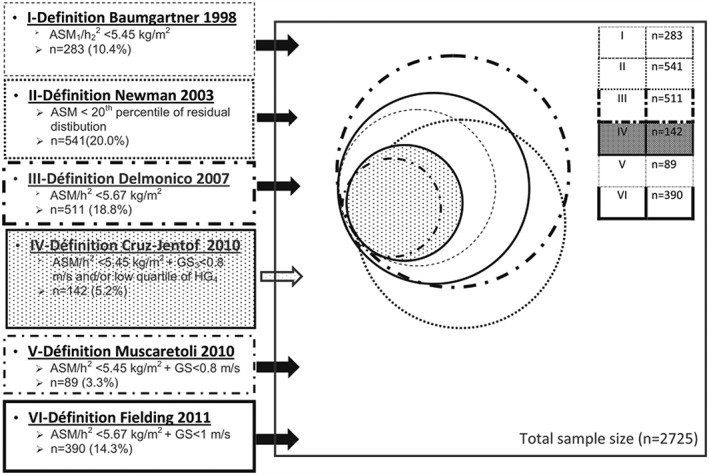
Distribution of a participant identified as sarcopenic according to the different definitions. ASM, appendicular skeletal muscle mass; h, height; GS, gait speed; HG, handgrip strength. Reproduced from Dupuy et al. 14
The easiest way of assessing sarcopenia seems to be bioelectrical impedance assessment for measuring lean mass coupled with either gait speed or handgrip dynamometry. An easy questionnaire is the SARC‐F questionnaire that is able to rapidly screen for sarcopenia, and the questionnaire's results have been found to be associated with physical performance, grip strength, and hospitalization in the previous 2 years.16 The SARC‐F questionnaire was studied in substudies of the African American Health Study, the Baltimore Longitudinal Study of Ageing, and the National Health and Nutrition Examination Survey. SARC‐F was evaluated here using cross‐sectional and longitudinal differences among those with high (≥4) vs. low (<4) SARC‐F scores for mortality and health outcomes. Overall, the SARC‐F questionnaire results proofed internally consistent and valid for detection of persons at risk for adverse outcomes from sarcopenia in the study cohorts investigated here.16 Estimates regarding the prevalence of sarcopenia using data from 18 363 people aged 65 years or above showed that sarcopenia was present 12.6% in Poland to up to 17.5% in India. The prevalence of sarcopenic obesity, a risk factor for impaired survival, for example, in cirrhosis,17 ranged from 1.3% in India to 11.0% in Spain. Low levels of physical activity were particularly associated with higher odds for the development of sarcopenia and sarcopenic obesity.18 Barbosa‐Silva et al.19 assessed the prevalence of sarcopenia as part of a cross‐sectional population‐based study in 1291 community‐dwelling elderly subjects aged 60 years or older using calf circumference measurements. Measurements were performed in the city of Pelotas in Brazil, and the authors found sarcopenia to be associated with low schooling level, being without a partner, low socio‐economic status, smoking, inactive life style, and low body mass index. The overall prevalence of sarcopenia was 13.9%.19 Foong et al. studied 636 community‐dwelling older adults whose muscle mass was measured using dual‐energy X‐ray absorptiometry (DEXA), and lower limb strength was measured via dynamometry.20 Not surprisingly, they found that physical activity intensity was positively associated with higher lean mass percentage and lower limb strength in a dose–response fashion. Sedentary activity was negatively associated with lean mass percentage but not with lower limb strength. This study underscores the importance of physical activity in elderly adults. In this context, handgrip strength remains one of the most importance screening tools, and a study by Leong et al. using data from 125 462 healthy adults from 21 countries aged 35–70 years showed that handgrip strength values differ among individuals from different geographic regions. Values were highest among subjects from Europe and North America but lowest from those from South Asia, South East Asia, and Africa and intermediate among those from China, South America, and the Middle East, buttressing the view that reference values need to adjust for ethnic backgrounds.21 The before mentioned data are in line with the results by Gielen et al.22 who showed that appendicular lean mass decreased from age 50 onwards, while gait speed and grip strength significantly decreased only from age 70. These authors used data from 518 men aged 40–79 years recruited for participation in the European Male Ageing Study, and they also found that the incidence of sarcopenia among their study cohort varied from 1.6% to 8.1% according to the definition of sarcopenia used. In men aged ≥70 years, low insulin‐like growth factor‐1 values were associated with a greater decrease in gait speed. However, baseline endocrine variables were not independently associated with an increased risk of incident sarcopenia by any definition.22 Finally, using data from a population‐based study cohort including 4425 older adults from the Third National Health and Nutrition Survey, Brown et al. found that the prevalence of sarcopenia was 36.5% and that sarcopenia was associated with an increased risk of all‐cause mortality in men and women; cardiovascular mortality was only increased in sarcopenic women.23
The problem of screening for the presence of sarcopenia, however, remains very valid, and several attempts have been made to establish a biomarker or a biomarker portfolio that could help in identifying patients with sarcopenia. C‐terminal Agrin Fragment (CAF) has been used in patients after acute stroke during early rehabilitation to identify those fulfilling criteria of sarcopenia.24 CAF originates from the degeneration of neuromuscular junctions, and its sub‐fragment CAF22 was elevated in patients admitted for rehabilitation after stroke. CAF22 values showed only incomplete recovery until discharge, and an association was found between CAF22 levels and parameters of physical performance, hand grip strength, and phase angle on bioelectrical impedance assessment measurement.24 Other markers that have been used as part of early studies include tartate‐resistant acid phosphatase 5a and epigallocatechin‐3‐gallate.25 However, the overall situation with regard to biomarkers remains unsatisfying, particularly concerning sensitivity and specificity.26 Nedergaard et al. studied blood samples and DEXA‐data from 41 patients with head and neck cancer as part of the Danish Head and Neck Cancer Group. They studied the collagen fragment serum markers Collagen type III propeptide (ProC3), Collagen type VI peptides containing the IC6 epitope, and Collagen type VI fragment C6M but were unable to show any correlation between biomarkers and lean body mass.27 It appears that these biomarkers, which worked as biomarkers of lean body mass in healthy individuals of both genders, do not work in the cancer patients investigated in this study. Because biomarkers have not emerged on the horizon in recent years, the focus remains on easily applicable muscle strength assessments such as handgrip strength and on questionnaires.
Treatment
At least three treatment approaches suggest themselves with regard to the treatment of sarcopenia. These include nutrition, physical activity, and pharmacotherapy or potentially a combination of these three. De Vries et al.28 performed patient‐centred physical therapy as part of a randomized controlled trial that included 13 physical therapy practices with follow‐up measurements at 3 and 6 months. Eligible patients to be included in the study were aged 70 years or over and had mobility problems. The primary outcome of the study was physical activity in minutes per day. The study included 130 patients, and the between‐group difference was significant for moderate intensity physical activity in favour of the ‘Coach2Move’ group that tested the effectiveness of patient‐centred physical therapy strategy. In this group, frailty also decreased more significantly than in the usual care group, and the overall results add to an improvement in the quality‐adjusted life years. Importantly, the authors reported that their approach using physical therapy was cost‐effective.28 Barbat‐Artigas and colleagues29 studied 146 obese women with a body mass index ≥ 30 kg/m2 who participated in a 3‐week usual and institutionalized weight‐reducing programme combined with a dietary plan and aerobic exercise. This therapeutic approach lead to loss of fat mass in both sarcopenic obese and non‐sarcopenic obese women, and it lead to an improvement in the lipid‐lipoprotein profile. However, lean mass index differences between the two groups remained significant even at the end of the weight‐reducing programme. The authors concluded that such treatment approaches may have deleterious effects on lean mass only in women who are non‐sarcopenic obese.29 Creatine supplementation has been used by Pinto et al.30 as part of a 12‐week double‐blind, randomized, placebo‐controlled trial in elderly subjects. Creatine supplementation plus resistance training was shown to have superior gains in lean mass when compared with the placebo group that also underwent resistance exercise training. However, this was not associated with an increase in the 10 repetition maximal tests in bench press and leg press exercise, which buttresses the view that increases in lean mass are not always associated with increases in strength, and ideal medication should combine an increase in both.30 Finally, a battle of ongoing debate remains the questions whether or not certain drugs might be associated with increases in muscle loss. Such discussion is, for example, ongoing with regard of using statins in elderly or terminally ill patients.31
Animal data
As outlined previously, changes in skeletal muscle structure and function are found in various clinical populations. However, the degradation of muscle fibres remains incompletely understood. The ubiquitin‐proteasome system plays a pivotal role in this context. The muscle specific RING‐finger (MuRF) protein family of E3 ligases is of particular importance in this context. Lodka et al. produced MuRF2 and MuRF3 double knock‐out mice and investigated skeletal muscle and heart by morphological measurements, histological analyses, electron microscopy, immunoblotting, and real‐time polymerase chain reaction. The authors found that affected mice showed a protein aggregate myopathy in skeletal muscle with reduced maximum force development and concluded that the redundant function of MuRF2 and MuRF3 is important for the maintenance of skeletal muscle and cardiac structure and function in vivo.32 Data by Nederveen et al. showed that a greater distance exists between capillaries and type II fibre‐associated skeletal muscle satellite cells suggesting implications in the adaptation of skeletal muscle to exercise.33 Likewise, mice with sarcopenia appear to have a marked defect of autophagy. Autophagy is part of the normal maintenance of muscle mass and function.34 Cationic influx into skeletal muscle via degenerine channel activation appears to cause caspase degradation of cytosolic proteins, which has been viewed as a result of mitochondrial dysfunction in ageing and sarcopenia.35
On a treatment level, recent data suggest that short‐term supplementation of docosahexaenoic acid may help in increasing energy stores and therefore in preserving muscle mass in response to fasting.36 Dihydrotestosterone treatment, on the other hand, was able to rescue the decline in protein synthesis in isolated skeletal muscle fibres from the mouse.37 The latter finding is important, because testosterone supplementation has been used in several clinical settings as part of smaller studies to preserve muscle mass and to increase functional capacity. Apart from the ubiquitin‐proteasome system and autophagy, the caspase system particularly involving apoptosis using Caspase‐3 seems to be another important trigger for age‐related muscle loss.38 Kob et al. found that male rats were more prone to the decline of muscle during ageing than female animals. This decline was further enhanced by long‐term high‐fat diet.39 Previous studies have reported that caloric restriction leads to an increase in the lifespan of rodents and might be able to reduce age‐induced muscle loss. Van Norren et al.40 performed a study in mice that received either an ad libitum diet (control) or a diet matching 70% of the intake of the control group. The authors then measured daily activity, body composition, grip strength, insulin sensitivity, and general agility and balance. Caloric‐restricted animals were found to have only half the weight of the control animals from the age of 12 months onwards. Interestingly, the control group showed a decline in hind limb muscle mass starting at the age of 24 or 28 months, which was not present in the caloric‐restriction group, and the study confirms that caloric‐restriction animals present with less sarcopenia prevalence than those eating ad libitum. The authors concluded that the positive effect of caloric restriction of muscle maintenance may not be only a direct consequence of lower energy intake but may also be related to more active behaviour in a specific time frame.40 Mogi et al. found that diabetes may enhance sarcopenic obesity development for a mechanism involving an anomalous fibro‐adipocyte progenitor cell differentiation.41 However, even though single studies can help us to understand the loss of skeletal muscle during the ageing process or during disease states, the list of mediating factors is very long including, for example, expression of protocadherin gamma.42 Other studies have shown beneficial effects on skeletal muscle mass or muscle performance using, for example, the metabolic modulator trimetazidine43 or the melanocortin receptor type 3 agonist d‐Trp(8)‐γMSH that decreases inflammation and muscle wasting in rats with arthritis.44
Heart failure
Cardiac cachexia as a consequence of advancing heart failure has been described as an independent risk factor of death already in 1997.45 Sarcopenia as a consequence of heart failure has only been described recently, and it was shown that patients with muscle wasting fulfilling the criteria of sarcopenia and heart failure have significantly reduced muscle strength and exercise capacity.46, 47 In patients with heart failure, muscle wasting can be approached by aerobic exercise training,48 which is being advocated for these patients irrespective of wasting being present or not as long as patients are clinically stable.49 Several other factors have been highlighted recently in the setting of heart failure. Using data from a rat model of heart failure developed after ligation of the left anterior decending coronary artery, it was shown that divergent antioxidant and metabolic responses exist in the diaphragm, and the quadriceps muscle with glutathione peroxidase and manganese superoxide dismutase activity increased in the diaphragm but reduced in the quadriceps.50 Metabolic enzymes were unaltered in the diaphragm, but cytochrome c oxidase activity decreased, and lactate dehydrogenase activity increased in the quadriceps of animals with heart failure. Catabolic responses remained similar between heart failure and non‐heart failure animals. In summary, MuRF‐1 and proteasome activity were elevated, and oxidative enzyme activity failed to increase in the diaphragm of animals with heart failure. This likely suggests that a myopathy is present in respiratory muscles of heart failure animals, which is interesting to note because this muscle remains in constant use.50, 51 In addition, adiponectin resistance has been recently reported in skeletal muscle of patients with chronic heart failure. Sente et al. proposed that the resistance process may be multifactorial and may lead to an integration of abnormalities from insulin signalling, mitochondrial biogenesis, and ceramide metabolism.52 Similarly, other factors have been involved in the development of skeletal muscle loss in patients with heart failure. In particular, the expression of skeletal muscle fibronectin type III domain containing 5 gene in patients with ischaemic cardiomyopathy as it appears that the reduced expression is modulated be inflammatory cytokines and angiotensin‐II. This factor may be involved in the slowing of browning of adipocytes.53 Rozentryt et al. showed that serum phosphorus is associated with a catabolic/anabolic imbalance in heart failure using data from 1029 stable patients of heart failure. These authors found that a catabolic/anabolic imbalance, N‐terminal pro‐B‐type natriuretic peptide values, serum sodium, kidney function, age, and sex were independent predictors of serum phosphorus. Thus, the authors concluded that metabolic status is an independent determinant of serum phosphorus in heart failure.54
Chronic obstructive pulmonary disease and mechanical ventilation
Cachexia and muscle wasting are common in the course of COPD. Both are recognized to have strong impact on disease progression and patients' prognosis.55 Recent evidence suggests that the expression of growth differentiation factor‐15 (GDF‐15) may be involved in the loss of skeletal muscle mass in patients with COPD. Patel et al.56 assessed the association of GDF‐15 expression with muscle mass and exercise performance and found increased serum values as well as increased expression in skeletal muscle in patients with COPD compared with controls. In particular, circulating GDF‐15 was inversely correlated with rectus femoris cross‐sectional area and exercise capacity. However, no association was found with body mass index. The authors therefore assume that GDF‐15 contributes to loss of muscle mass in patients with COPD.56 Lewis et al.57 identified microRNAs associated with low fat‐free mass phenotype in patients with COPD using a polymerase chain reaction screen of 750 microRNAs. They found that imprinted miR‐675 and a cluster including miR‐519a were differentially expressed in the quadriceps of patients with low fat‐free mass index compared with those with normal values. In particular, the association of miR‐519a expression with fat‐free mass index was present in male patients with severe COPD, and in vitro miR‐675 inhibited myoblast proliferation. The authors therefore concluded that increased expression of miR‐675 and also altered methylation of the H19 imprinting control region are associated with a low fat‐free mass index in patients with COPD.57 Just like in patients with COPD, muscle weakness and muscle wasting are frequently observed in critically ill patients on the intensive care unit. These patients are at particular risk because such muscle wasting may be involved in the failure of weaning from mechanical ventilation.58
Cancer
Pathophysiology
Cachexia development in patients with cancer is of a multi‐dimension nature. The prevalence varies strongly according to tumour type between 28% in patients with colorectal cancer and 57% or more in patients with head and neck or pancreatic cancer.59, 60 Yet still cachexia remains under recognized.61 The experience of weight loss differs between patients and care givers.62 The multi‐dimensional aspect of wasting in patients with cancer is buttressed by the fact that anorexia is commonly present in patients with cancer and a major component of cancer cachexia. Anorexia in itself has multiple causes such as substance‐release from the tumour (e.g. pro‐inflammatory cytokines), dysphagia caused by the tumour itself, the alteration of nutrients by the tumour (e.g. zinc deficiency), tumours causing tissue hypoxia, increased accumulation of serotonin by increased peripheral tryptophan amounts, or the alterations of the release of peripheral hormones that alter feeding.63 Even though an understanding of anorexia pathophysiology is being developed, therapeutic approaches in this regard remain unsatisfying. Different drugs such as megestrole acetate, cannabinoids, and ghrelin agonists have been used with mixed effectiveness at best. Another phenomenon commonly encountered in patients with cachexia is hypermetabolism. Dev et al. studied 60 patients with advanced cancer and weight loss exceeding 5% or anorexia who underwent indirect calorimetry to measure resting energy expenditure. Most patients had a solid tumour, predominately gastrointestinal cancers. Hypermetabolism was present in 58% of the patients in the study, buttressing the view that this phenomenon is very frequent in patients with cancer.64 However, it remains unclear whether hypermetabolism might be an appealing therapeutic target.65 Moryoussef et al. studied 31 patients with advanced or high‐risk resected gastrointestinal stroma tumours and found that 12 of these patients were sarcopenic. Approximately 63.6% of the assessable sarcopenic patients became non‐sarcopenic after 6 months of imatinib therapy, and the authors therefore conclude that sarcopenia might be reversible in some patients with gastrointestinal stroma tumours who were treated with imatinib.66
Prognosis
Several biomarkers have been used in body wasting to predict outcomes and survival. Recent evidence shows that GDF‐15 levels, a marker that has been used several times,67 were increased in patients with cancer‐related weight loss just like interleukin‐6 and interleukin‐8.68 This was also true for interleukin‐1 receptor antagonist (IL‐1RA), interleukin‐4, interferon‐γ, and tumour necrosis factor. Patients with cancer‐related weight loss had lower handgrip strength, lower appendicular lean body mass, and fat mass as well as a lower Eastern Cooperative Oncology Group and Karnofsky performance scores. Lerner et al. found that GDF‐15, interleukin‐6, and interleukin‐8 correlated significantly with weight loss, and in particular, GDF‐15 was negatively correlated with appendicular lean body mass, hand grip strength, and fat mass. The authors suggest that GDF‐15 may serve as a prognostic indicator in patients with cancer.68 Stephens et al. studied 107 subjects, 92 of whom had upper gastrointestinal cancer, and the remaining were controls. Rectus abdominis muscle specimens were obtained at surgery and analysed with regard to the expression of Akt and phosphorylated Akt as well as forkhead box O (FOXO) transcription factors, ubiquitin E3 ligases, BIP3, and GABARAPL1 as well as other markers, particularly myosin heavy chain and dystrophin. The latter two markers were able to predict survival in patients with lower levels of myosin heavy chain and dystrophin.69 As discussed earlier, there is poor sensitivity and specificity for biomarkers in any type of wasting whether cachexia or sarcopenia. Nyasavajjala et al.70 evaluated creatinine and myoglobin as predictors of anaerobic threshold in patients with colorectal cancer. For this purpose, they recruited 47 patients with colorectal cancer and a matching number of healthy volunteers and assessed their body composition using DEXA scan and their exercise capacity using the measurement of anaerobic threshold on spiroergometry. They found that in spite of differences in the aerobic capacity, no differences were found with regard to the serum levels of myoglobin and creatinine between the two groups. However, anaerobic threshold was significantly lower in the colorectal cancer group as compared with the control group, and there was a significant correlation between lean muscle mass and anaerobic threshold. No such correlation was found with regard to myoglobin and creatinine, and the authors therefore conclude that their predicted value remains low.70
Treatment
Exercise training has been advocated in recent years as having positive effects on exercise capacity and overall well‐being, quality of life, and potentially even of prognosis. Exercise training is one of the mainstays in the maintenance of muscle mass in patients with cachexia or sarcopenia as long as such approach is possible from a physical point of view for the patient. A recent Cochrane Collaboration systematic review showed disappointing results after screening more than 3000 separate manuscripts, because most authors who did explore the concept of exercise training did so only in patients with cancer but not in cancer cachexia.71 Still, a small number of intervention studies have investigated the impact of exercise training in patients with cancer cachexia. Sasso et al.72 have provided intriguing insight and suggest a framework for the prescription of exercise in patients with cancer (Figure 2). They concluded that the vast majority of studies have tested the efficacy of an exercise prescription that adhered to traditional guidelines consisting of either supervised or home‐based endurance training or endurance training combined with resistance training. The prescription in these studies that did not target cachexia patients but rather oncology patients per se was typically prescribed at a moderate intensity of 50–75% of predetermined physiological parameters, typically age‐predicted heart rate maximum or reserve. The usual prescription frequency was 2–3 sessions per week of 10‐ to 60‐min duration, and the usual time frame was 12–15 weeks.72
Figure 2.
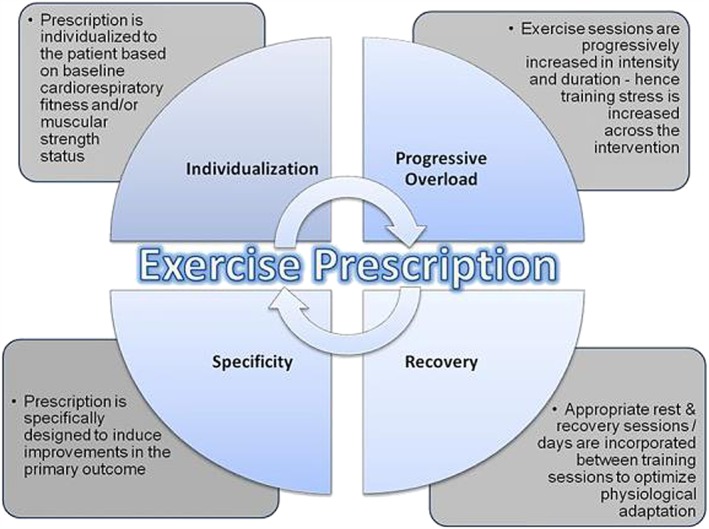
The principles of training. Reproduced from Sasso et al. 72
As mentioned before, there are, at present, no therapeutic interventions being established for the treatment of muscle wasting and sarcopenia or cachexia. However, exercise training is known to exert anti‐inflammatory and anti‐oxidative effects that are able to attenuate signalling pathways associated with protein degradation, which renders this therapeutic concept very interesting.73 Such pathways include, for example, muscle regulatory factors, which were studied by Slimani et al.74 using a tibialis anterior muscle atrophy model in rats that were subjected to hindlimb cast immobilization for 8 days. They found that remobilization correlated with reduced fibre cross‐sectional area and thickening of the endomysium. Integrin‐linked kinase protein levels increased during immobilization and remobilization after 10 days, and this was inversely correlated with changes in Akt phosphorylation. The authors concluded that tibialis anterior muscle atrophy can be counterbalanced during remobilization only when the terminal differentiation step of regeneration is enhanced.74 Indeed, the number of pathways and players involved in the development of muscle atrophy and sarcopenia is increasing steeply, and it is sometimes difficult to keep track. Marino et al.75 studied increased levels of atrogin‐1, MuRF‐1, Beclin‐1 p62, Smad‐2, and activin‐A in a mouse model of muscle wasting. Overexpression of activin‐βC antagonized the ubiquitin‐proteasome system activity and the autophagic‐lysosomal degradation pathways yielding reduction in the serum levels of activin‐A. The authors therefore suggest that activin‐βC may be a novel therapy to abrogate cancer‐associated weight loss and potentially even prolonging survival.75
From a clinical standpoint, nutritional approaches as well as pharmacological interventions remain important.76 Stewart Coats et al.77 studied 87 patients with stage III or IV colorectal cancer or non‐small cell lung cancer who were cachetic and received one of two doses of espindolol or placebo. Espindolol is a novel non‐selective beta‐blocker with central 5‐HT1a and partial β2 receptor agonist effects. After 16 weeks of treatment, patients on high‐dose espindolol showed a significant increase in lean body mass and handgrip strength. Stair climbing power and 6‐min walk distance remained statistically non‐significant but showed an effect favouring high‐dose espindolol. Overall, espindolol was well‐tolerated, and the authors suggest to study the drug in a larger cohort of patients.77, 78 Besides exercise capacity, fatigue is an important problem affecting patients under palliative care. A recent Cochrane Collaboration systematic review screened more than 1600 publications using data from 18 drugs and almost 4700 participants. Unfortunately, the authors concluded that because of the heterogeneity of the trials, they could not recommend a specific drug for the treatment of fatigue in palliative care patients. However, the authors highlighted amantadine, methylphenidate, and modafinil to be further researched in larger settings.79 A similar standpoint has been advocated for anamorelin.80 On the other hand, a lack of regulatory guidance has been deplored, as it is not entirely clear what kind of endpoints in clinical trials of cachexia will be acceptable for regulatory bodies such as the Food and Drug Administration or the European Medicines Agency.81
From a therapeutic perspective, some studies also tested the effect of chemotherapy regimens on muscle wasting. Rutten et al.82 found that patients with ovarian cancer have worst survival when they loose skeletal muscle during neoadjuvant chemotherapy. However, their study remained small with only 123 ovarian cancer patients treated with neoadjuvant chemotherapy and interval debulking in the Netherlands. The proteasome inhibitor bortezomib was studied only in an animal model of muscle wasting in cancer cachexia that was induced in rats by i.p. injection of a hepatoma cell line.83 Even though bortezomib administration reduced proteasome and nuclear factor‐κB activity in skeletal muscle after tumour transplantation, this effect did not prevent body weight loss and muscle wasting. The authors concluded that further studies are needed to address this issue. Toledo et al. studied a multifactorial treatment design including formoterol and megestrol acetate in cachectic tumour‐bearing rats undergoing chemotherapy with sorafenib. Sorafenib is known to decrease tumour cell content; however, cachexia occurrence in affected animals persists. The authors found that the combination of formoterol and megestrol acetate may be a promising strategy for treating cancer cachexia in a preclinical setting and suggest that a clinical trial involving cachectic cancer patients may be warranted.84 Another study investigated the effects of megestrol acetate in improving cardiac function in a model of cancer cachexia‐induced cardiomyopathy by autophagic modulation.85 The authors found that the use of megestrol acetate, an appetite stimulating, improves the survival and reduces wasting through a marked down‐regulation of autophagy, both in skeletal and cardiac muscle. Particularly, the latter effect leads to a significant improvement in cardiac function.85 Chen et al.86 found that ghrelin prevents tumour‐induced and cisplatin‐induced muscle wasting. They demonstrated that implantation of a Lewis lung carcinoma cell line into adult male mice together with the use of cisplatin‐induced muscle atrophy by activating pro‐inflammatory cytokines, myostatin, and p38‐C/EBP‐β and by down‐regulating Akt, myoD, and myogenin altogether leading to an activation of the ubiquitin‐proteasome system. Ghrelin was able to prevent these changes in vivo and in vitro by significantly increasing muscle mass and grip strength as well as by improving survival. In this context, it is interesting to note that the diet composition may be a source of variation in animal models of cancer cachexia. Giles et al.87 recommend that minimum standards should be used for diet definition in models of cachexia, which includes the reporting of diet content and composition as well as food intake to improve reproducibility of preclinical studies. Likewise, Dwarkasing et al.88 found that altered serotonin signalling is associated with changes in food intake behaviour in cachectic mice and suggest that serotonin regulation might be a therapeutic target to prevent the development of cancer‐induced eating disorders. Because essential amino acids are indeed an essential component of muscle structure, particularly the branched‐chain amino acids valine, isoleucine, and leucine, a study by Toneto et al.89 put a particular focus on nutritional leucine supplementation. They found that a leucine‐rich diet could modulate heart damage, cardiomyocyte proteolysis, and apoptosis driven by cancer cachexia in a model of male rats that were inoculated with a subcutaneous Walker‐256 carcinoma cell line. All in all, it appears that nutrition screening is important as well as nutritional supplements in the treatment of patients with cachexia—particularly in those with cancer.90 Having said this, the importance of also screening for severe dysphagia cannot be stressed enough. Wakabayashi et al. found skeletal muscle to be associated with severe dysphagia in patients with cancer.91, 92 However, it remains important that not only patients are affected by eating disorders or eating‐related distress but this can also involve bereaved family members. Amano et al.93 performed a study in 133 inpatient hospices in Japan involving a total of 702 bereaved family members and found that family members of advanced cancer patients may experience high levels of eating‐related distress and may require nutritional counselling.
Conclusions
The aforementioned text describes advances in the field of cachexia, sarcopenia, and wasting research. It is reassuring that reasonable progress is being made; however, it is also quite disappointing that this progress is made at such low speed. We have a huge number of biomarker candidates for the diagnosis and prognosis of muscle wasting, but none is ready for clinical use. We have numerous candidate drugs, but none is ready for clinical use unless one may count megestrol acetate that has approval only in the setting of breast cancer. The aforementioned text also describes one of the major problems that we are facing in cachexia and wasting research: the lack of randomized controlled trials of reasonable size. Therefore, we are—for the time being—left with nutritional counselling and the use of exercise training. Some hope remains that the Journal of Cachexia, Sarcopenia and Muscle will soon report novel insight into pathophysiology and—most importantly—treatments of wasting disorders.
Conflict of interest
None declared.
Acknowledgements
The author of this manuscript certifies that he complies with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle.94
von Haehling, S. (2017) Casting the net broader to confirm our imaginations: the long road to treating wasting disorders. Journal of Cachexia, Sarcopenia and Muscle, 8: 870–880. doi: 10.1002/jcsm.12256.
References
- 1. Santilli V, Bernetti A, Mangone M, Paoloni M. Clinical definition of sarcopenia. Clin Cases Miner Bone Metab 2014;11:177–180. [PMC free article] [PubMed] [Google Scholar]
- 2. Szulc P, Feyt C, Chapurlat R. High risk of fall, poor physical function, and low grip strength in men with fracture‐the STRAMBO study. J Cachexia Sarcopenia Muscle 2016;7:299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ebner N, von Haehling S. Unlocking the wasting enigma: highlights from the 8th Cachexia Conference. J Cachexia Sarcopenia Muscle 2016;7:90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. von Haehling S, Anker SD. The times they are a‐changin’: the Cachexia Conference goes annual. J Cachexia Sarcopenia Muscle 2016;7:3–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. von Haehling S, Ebner N, Anker SD. Moving upwards – the Journal of Cachexia, Sarcopenia and Muscle in 2016. J Cachexia Sarcopenia Muscle 2016;7:391–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. von Haehling S, Anker SD. More colour to the journal: new style, new publisher, but still Cachexia, Sarcopenia and Muscle. J Cachexia Sarcopenia Muscle 2015;6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Anker SD, Morley JE, von Haehling S. Welcome to the ICD‐10 code for sarcopenia. J Cachexia Sarcopenia Muscle 2016;7:512–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Manini TM, Clark BC. Letter to the editor: results from a web‐based survey to identify dynapenia screening tools and risk factors. J Cachexia Sarcopenia Muscle 2016;7:499–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morley JE, Abbatecola AM, Argiles JM, Baracos V, Bauer J, Bhasin S, Cederholm T, Coats AJ, Cummings SR, Evans WJ, Fearon K, Ferrucci L, Fielding RA, Guralnik JM, Harris TB, Inui A, Kalantar‐Zadeh K, Kirwan BA, Mantovani G, Muscaritoli M, Newman AB, Rossi‐Fanelli F, Rosano GM, Roubenoff R, Schambelan M, Sokol GH, Storer TW, Vellas B, von Haehling S, Yeh SS, Anker SD, Society on Sarcopenia, Cachexia and Wasting Disorders Trialist Workshop . Sarcopenia with limited mobility: an international consensus. J Am Med Dir Assoc 2011;12:403–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Muscaritoli M, Anker SD, Argilés J, Aversa Z, Bauer JM, Biolo G, Boirie Y, Bosaeus I, Cederholm T, Costelli P, Fearon KC, Laviano A, Maggio M, Rossi Fanelli F, Schneider SM, Schols A, Sieber CC. Consensus definition of sarcopenia, cachexia and pre‐cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia‐anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr 2010;29:154–159. [DOI] [PubMed] [Google Scholar]
- 11. Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D, Cederholm T, Chandler J, De Meynard C, Donini L, Harris T, Kannt A, Keime Guibert F, Onder G, Papanicolaou D, Rolland Y, Rooks D, Sieber C, Souhami E, Verlaan S, Zamboni M. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International Working Group on Sarcopenia. J Am Med Dir Assoc 2011;12:249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M, European Working Group on Sarcopenia in Older People . Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dam TT, Peters KW, Fragala M, Cawthon PM, Harris TB, McLean R, Shardell M, Alley DE, Kenny A, Ferrucci L, Guralnik J, Kiel DP, Kritchevsky S, Vassileva MT, Studenski S. An evidence‐based comparison of operational criteria for the presence of sarcopenia. J Gerontol A Biol Sci Med Sci 2014;69:584–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dupuy C, Lauwers‐Cances V, Guyonnet S, Gentil C, Abellan Van Kan G, Beauchet O, Schott AM, Vellas B, Rolland Y. Searching for a relevant definition of sarcopenia: results from the cross‐sectional EPIDOS study. J Cachexia Sarcopenia Muscle 2015;6:144–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Morley JE, Cao L. Rapid screening for sarcopenia. J Cachexia Sarcopenia Muscle 2015;6:312–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Malmstrom TK, Miller DK, Simonsick EM, Ferrucci L, Morley JE. SARC‐F: a symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J Cachexia Sarcopenia Muscle 2016;7:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Montano‐Loza AJ, Angulo P, Meza‐Junco J, Prado CM, Sawyer MB, Beaumont C, Esfandiari N, Ma M, Baracos VE. Sarcopenic obesity and myosteatosis are associated with higher mortality in patients with cirrhosis. J Cachexia Sarcopenia Muscle 2016;7:126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tyrovolas S, Koyanagi A, Olaya B, Ayuso‐Mateos JL, Miret M, Chatterji S, Tobiasz‐Adamczyk B, Koskinen S, Leonardi M, Haro JM. Factors associated with skeletal muscle mass, sarcopenia, and sarcopenic obesity in older adults: a multi‐continent study. J Cachexia Sarcopenia Muscle 2016;7:312–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barbosa‐Silva TG, Bielemann RM, Gonzalez MC, Menezes AM. Prevalence of sarcopenia among community‐dwelling elderly of a medium‐sized South American city: results of the COMO VAI? study. J Cachexia Sarcopenia Muscle 2016;7:136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Foong YC, Chherawala N, Aitken D, Scott D, Winzenberg T, Jones G. Accelerometer‐determined physical activity, muscle mass, and leg strength in community‐dwelling older adults. J Cachexia Sarcopenia Muscle 2016;7:275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leong DP, Teo KK, Rangarajan S, Kutty VR, Lanas F, Hui C, Quanyong X, Zhenzhen Q, Jinhua T, Noorhassim I, AlHabib KF, Moss SJ, Rosengren A, Akalin AA, Rahman O, Chifamba J, Orlandini A, Kumar R, Yeates K, Gupta R, Yusufali A, Dans A, Avezum Á, Lopez‐Jaramillo P, Poirier P, Heidari H, Zatonska K, Iqbal R, Khatib R, Yusuf S. Reference ranges of handgrip strength from 125,462 healthy adults in 21 countries: a prospective urban rural epidemiologic (PURE) study. J Cachexia Sarcopenia Muscle 2016;7:535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gielen E, O'Neill TW, Pye SR, Adams JE, Wu FC, Laurent MR, Claessens F, Ward KA, Boonen S, Bouillon R, Vanderschueren D, Verschueren S. Endocrine determinants of incident sarcopenia in middle‐aged and elderly European men. J Cachexia Sarcopenia Muscle 2015;6:242–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brown JC, Harhay MO, Harhay MN. Sarcopenia and mortality among a population‐based sample of community‐dwelling older adults. J Cachexia Sarcopenia Muscle 2016;7:290–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Scherbakov N, Knops M, Ebner N, Valentova M, Sandek A, Grittner U, Dahinden P, Hettwer S, Schefold JC, von Haehling S, Anker SD, Joebges M, Doehner W. Evaluation of C‐terminal agrin fragment as a marker of muscle wasting in patients after acute stroke during early rehabilitation. J Cachexia Sarcopenia Muscle 2016;7:60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Drescher C, Konishi M, Ebner N, Springer J. Loss of muscle mass: current developments in cachexia and sarcopenia focused on biomarkers and treatment. J Cachexia Sarcopenia Muscle 2015;6:303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Calvani R, Marini F, Cesari M, Tosato M, Anker SD, von Haehling S, Miller RR, Bernabei R, Landi F, Marzetti E, SPRINTT consortium . Biomarkers for physical frailty and sarcopenia: state of the science and future developments. J Cachexia Sarcopenia Muscle 2015;6:278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nedergaard A, Dalgas U, Primdahl H, Johansen J, Overgaard J, Overgaard K, Henriksen K, Karsdal MA, Lønbro S. Collagen fragment biomarkers as serological biomarkers of lean body mass – a biomarker pilot study from the DAHANCA25B cohort and matched controls. J Cachexia Sarcopenia Muscle 2015;6:335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. de Vries NM, Staal JB, van der Wees PJ, Adang EM, Akkermans R, Olde Rikkert MG, Nijhuis‐van der Sanden MW. Patient‐centred physical therapy is (cost‐) effective in increasing physical activity and reducing frailty in older adults with mobility problems: a randomized controlled trial with 6 months follow‐up. J Cachexia Sarcopenia Muscle 2016;7:422–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Barbat‐Artigas S, Garnier S, Joffroy S, Riesco É, Sanguignol F, Vellas B, Rolland Y, Andrieu S, Aubertin‐Leheudre M, Mauriège P. Caloric restriction and aerobic exercise in sarcopenic and non‐sarcopenic obese women: an observational and retrospective study. J Cachexia Sarcopenia Muscle 2016;7:284–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pinto CL, Botelho PB, Carneiro JA, Mota JF. Impact of creatine supplementation in combination with resistance training on lean mass in the elderly. J Cachexia Sarcopenia Muscle 2016;7:413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Banach M, Serban MC. Discussion around statin discontinuation in older adults and patients with wasting diseases. J Cachexia Sarcopenia Muscle 2016;7:396–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lodka D, Pahuja A, Geers‐Knörr C, Scheibe RJ, Nowak M, Hamati J, Köhncke C, Purfürst B, Kanashova T, Schmidt S, Glass DJ, Morano I, Heuser A, Kraft T, Bassel‐Duby R, Olson EN, Dittmar G, Sommer T, Fielitz J. Muscle RING‐finger 2 and 3 maintain striated‐muscle structure and function. J Cachexia Sarcopenia Muscle 2016;7:165–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nederveen JP, Joanisse S, Snijders T, Ivankovic V, Baker SK, Phillips SM, Parise G. Skeletal muscle satellite cells are located at a closer proximity to capillaries in healthy young compared with older men. J Cachexia Sarcopenia Muscle 2016;7:547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sakuma K, Kinoshita M, Ito Y, Aizawa M, Aoi W, Yamaguchi A. p62/SQSTM1 but not LC3 is accumulated in sarcopenic muscle of mice. J Cachexia Sarcopenia Muscle 2016;7:204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gaffney CJ, Shephard F, Chu J, Baillie DL, Rose A, Constantin‐Teodosiu D, Greenhaff PL, Szewczyk NJ. Degenerin channel activation causes caspase‐mediated protein degradation and mitochondrial dysfunction in adult C. elegans muscle. J Cachexia Sarcopenia Muscle 2016;7:181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Deval C, Capel F, Laillet B, Polge C, Béchet D, Taillandier D, Attaix D, Combaret L. Docosahexaenoic acid‐supplementation prior to fasting prevents muscle atrophy in mice. J Cachexia Sarcopenia Muscle 2016;7:587–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wendowski O, Redshaw Z, Mutungi G. Dihydrotestosterone treatment rescues the decline in protein synthesis as a result of sarcopenia in isolated mouse skeletal muscle fibres. J Cachexia Sarcopenia Muscle 2017;8:48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fielitz J. Cancer cachexia – when proteasomal inhibition is not enough. J Cachexia Sarcopenia Muscle 2016;7:239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kob R, Fellner C, Bertsch T, Wittmann A, Mishura D, Sieber CC, Fischer BE, Stroszczynski C, Bollheimer CL. Gender‐specific differences in the development of sarcopenia in the rodent model of the ageing high‐fat rat. J Cachexia Sarcopenia Muscle 2015;6:181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. van Norren K, Rusli F, van Dijk M, Lute C, Nagel J, Dijk FJ, Dwarkasing J, Boekschoten MV, Luiking Y, Witkamp RF, Müller M, Steegenga WT. Behavioural changes are a major contributing factor in the reduction of sarcopenia in caloric‐restricted ageing mice. J Cachexia Sarcopenia Muscle 2015;6:253–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mogi M, Kohara K, Nakaoka H, Kan‐No H, Tsukuda K, Wang XL, Chisaka T, Bai HY, Shan BS, Kukida M, Iwanami J, Miki T, Horiuchi M. Diabetic mice exhibited a peculiar alteration in body composition with exaggerated ectopic fat deposition after muscle injury due to anomalous cell differentiation. J Cachexia Sarcopenia Muscle 2016;7:213–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hangelbroek RW, Fazelzadeh P, Tieland M, Boekschoten MV, Hooiveld GJ, van Duynhoven JP, Timmons JA, Verdijk LB, de Groot LC, van Loon LJ, Müller M. Expression of protocadherin gamma in skeletal muscle tissue is associated with age and muscle weakness. J Cachexia Sarcopenia Muscle 2016;7:604–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ferraro E, Pin F, Gorini S, Pontecorvo L, Ferri A, Mollace V, Costelli P, Rosano G. Improvement of skeletal muscle performance in ageing by the metabolic modulator Trimetazidine. J Cachexia Sarcopenia Muscle 2016;7:449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gómez‐SanMiguel AB, Martín AI, Nieto‐Bona MP, Fernández‐Galaz C, Villanúa MÁ, López‐Calderón A. The melanocortin receptor type 3 agonist d‐Trp(8)‐γMSH decreases inflammation and muscle wasting in arthritic rats. J Cachexia Sarcopenia Muscle 2016;7:79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Anker SD, Ponikowski P, Varney S, Chua TP, Clark AL, Webb‐Peploe KM, Harrington D, Kox WJ, Poole‐Wilson PA, Coats AJ. Wasting as independent risk factor for mortality in chronic heart failure. Lancet 1997;349:1050–1053. [DOI] [PubMed] [Google Scholar]
- 46. Fülster S, Tacke M, Sandek A, Ebner N, Tschöpe C, Doehner W, Anker SD, von Haehling S. Muscle wasting in patients with chronic heart failure: results from the Studies Investigating Co‐morbidities Aggravating Heart Failure (SICA‐HF). Eur Heart J 2013;34:512–519. [DOI] [PubMed] [Google Scholar]
- 47. Bekfani T, Pellicori P, Morris DA, Ebner N, Valentova M, Steinbeck L, Wachter R, Elsner S, Sliziuk V, Schefold JC, Sandek A, Doehner W, Cleland JG, Lainscak M, Anker SD, von Haehling S. Sarcopenia in patients with heart failure with preserved ejection fraction: Impact on muscle strength, exercise capacity and quality of life. Int J Cardiol 2016;222:41–46. [DOI] [PubMed] [Google Scholar]
- 48. Loncar G, Springer J, Anker M, Doehner W, Lainscak M. Cardiac cachexia: hic et nunc. J Cachexia Sarcopenia Muscle 2016;7:246–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. von Haehling S, Ebner N, Dos Santos MR, Springer J, Anker SD. Muscle wasting and cachexia in heart failure: mechanisms and therapies. Nat Rev Cardiol 2017;14:323–341. [DOI] [PubMed] [Google Scholar]
- 50. Mangner N, Weikert B, Bowen TS, Sandri M, Höllriegel R, Erbs S, Hambrecht R, Schuler G, Linke A, Gielen S, Adams V. Skeletal muscle alterations in chronic heart failure: differential effects on quadriceps and diaphragm. J Cachexia Sarcopenia Muscle 2015;6:381–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Konishi M, Ishida J, Saitoh M, Springer J. Clinical perspective for wasting in diaphragm, an ever‐trained muscle. J Cachexia Sarcopenia Muscle 2016;7:497–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sente T, Van Berendoncks AM, Hoymans VY, Vrints CJ. Adiponectin resistance in skeletal muscle: pathophysiological implications in chronic heart failure. J Cachexia Sarcopenia Muscle 2016;7:261–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Matsuo Y, Gleitsmann K, Mangner N, Werner S, Fischer T, Bowen TS, Kricke A, Matsumoto Y, Kurabayashi M, Schuler G, Linke A, Adams V. Fibronectin type III domain containing 5 expression in skeletal muscle in chronic heart failure‐relevance of inflammatory cytokines. J Cachexia Sarcopenia Muscle 2015;6:62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rozentryt P, Niedziela JT, Hudzik B, Lekston A, Doehner W, Jankowska EA, Nowak J, von Haehling S, Partyka R, Rywik T, Anker SD, Ponikowski P, Poloński L. Higher serum phosphorus is associated with catabolic/anabolic imbalance in heart failure. J Cachexia Sarcopenia Muscle 2015;6:325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sanders KJ, Kneppers AE, van de Bool C, Langen RC, Schols AM. Cachexia in chronic obstructive pulmonary disease: new insights and therapeutic perspective. J Cachexia Sarcopenia Muscle 2016;7:5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Patel MS, Lee J, Baz M, Wells CE, Bloch S, Lewis A, Donaldson AV, Garfield BE, Hopkinson NS, Natanek A, Man WD, Wells DJ, Baker EH, Polkey MI, Kemp PR. Growth differentiation factor‐15 is associated with muscle mass in chronic obstructive pulmonary disease and promotes muscle wasting in vivo. J Cachexia Sarcopenia Muscle 2016;7:436–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lewis A, Lee JY, Donaldson AV, Natanek SA, Vaidyanathan S, Man WD, Hopkinson NS, Sayer AA, Patel HP, Cooper C, Syddall H, Polkey MI, Kemp PR. Increased expression of H19/miR‐675 is associated with a low fat‐free mass index in patients with COPD. J Cachexia Sarcopenia Muscle 2016;7:330–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Berger D, Bloechlinger S, von Haehling S, Doehner W, Takala J, Z'Graggen WJ, Schefold JC. Dysfunction of respiratory muscles in critically ill patients on the intensive care unit. J Cachexia Sarcopenia Muscle 2016;7:403–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lees J. Incidence of weight loss in head and neck cancer patients on commencing radiotherapy treatment at a regional oncology centre. Eur J Cancer Care 1999;8:133–136. [DOI] [PubMed] [Google Scholar]
- 60. Dewys WD, Begg C, Lavin PT, Band PR, Bennett JM, Bertino JR, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med 1980;69:491–497. [DOI] [PubMed] [Google Scholar]
- 61. Laviano A, Molfino A. Cachexia: looking yet not seeing. J Cachexia Sarcopenia Muscle 2016;7:510–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cooper C, Burden ST, Cheng H, Molassiotis A. Understanding and managing cancer‐related weight loss and anorexia: insights from a systematic review of qualitative research. J Cachexia Sarcopenia Muscle 2015;6:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ezeoke CC, Morley JE. Pathophysiology of anorexia in the cancer cachexia syndrome. J Cachexia Sarcopenia Muscle 2015;6:287–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Dev R, Hui D, Chisholm G, Delgado‐Guay M, Dalal S, Del Fabbro E, Bruera E. Hypermetabolism and symptom burden in advanced cancer patients evaluated in a cachexia clinic. J Cachexia Sarcopenia Muscle 2015;6:95–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ishida J, Konishi M, Saito M, Springer J. Hypermetabolism: should cancer types, pathological stages and races be considered in assessing metabolism and could elevated resting energy expenditure be the therapeutic target in patients with advanced cancer? J Cachexia Sarcopenia Muscle 2015;6:391–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Moryoussef F, Dhooge M, Volet J, Barbe C, Brezault C, Hoeffel C, Coriat R, Bouché O. Reversible sarcopenia in patients with gastrointestinal stromal tumor treated with imatinib. J Cachexia Sarcopenia Muscle 2015;6:343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ishida J, Konishi M, Saitoh M, Springer J. Growth differentiation factor‐15 as a prognostic biomarker in cancer patients. J Cachexia Sarcopenia Muscle 2016;7:235–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lerner L, Hayes TG, Tao N, Krieger B, Feng B, Wu Z, Nicoletti R, Chiu MI, Gyuris J, Garcia JM. Plasma growth differentiation factor 15 is associated with weight loss and mortality in cancer patients. J Cachexia Sarcopenia Muscle 2015;6:317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Stephens NA, Skipworth RJ, Gallagher IJ, Greig CA, Guttridge DC, Ross JA, Fearon KC. Evaluating potential biomarkers of cachexia and survival in skeletal muscle of upper gastrointestinal cancer patients. J Cachexia Sarcopenia Muscle 2015;6:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Nyasavajjala SM, Phillips BE, Lund JN, Williams JP. Creatinine and myoglobin are poor predictors of anaerobic threshold in colorectal cancer and health. J Cachexia Sarcopenia Muscle 2015;6:125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Grande AJ, Silva V, Maddocks M. Exercise for cancer cachexia in adults: executive summary of a Cochrane Collaboration systematic review. J Cachexia Sarcopenia Muscle 2015;6:208–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sasso JP, Eves ND, Christensen JF, Koelwyn GJ, Scott J, Jones LW. A framework for prescription in exercise‐oncology research. J Cachexia Sarcopenia Muscle 2015;6:115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bowen TS, Schuler G, Adams V. Skeletal muscle wasting in cachexia and sarcopenia: molecular pathophysiology and impact of exercise training. J Cachexia Sarcopenia Muscle 2015;6:197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Slimani L, Vazeille E, Deval C, Meunier B, Polge C, Dardevet D, Béchet D, Taillandier D, Micol D, Listrat A, Attaix D, Combaret L. The delayed recovery of the remobilized rat tibialis anterior muscle reflects a defect in proliferative and terminal differentiation that impairs early regenerative processes. J Cachexia Sarcopenia Muscle 2015;6:73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Marino FE, Risbridger G, Gold E. Activin‐βC modulates cachexia by repressing the ubiquitin‐proteasome and autophagic degradation pathways. J Cachexia Sarcopenia Muscle 2015;6:365–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Konishi M, Ishida J, von Haehling S, Anker SD, Springer J. Nutrition in cachexia: from bench to bedside. J Cachexia Sarcopenia Muscle 2016;7:107–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Stewart Coats AJ, Ho GF, Prabhash K, von Haehling S, Tilson J, Brown R, Beadle J, Anker SD; for and on behalf of the ACT‐ONE study group . Espindolol for the treatment and prevention of cachexia in patients with stage III/IV non‐small cell lung cancer or colorectal cancer: a randomized, double‐blind, placebo‐controlled, international multicentre phase II study (the ACT‐ONE trial). J Cachexia Sarcopenia Muscle 2016;7:355–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lainscak M, Laviano A. ACT‐ONE – ACTION at last on cancer cachexia by adapting a novel action beta‐blocker. J Cachexia Sarcopenia Muscle 2016;7:400–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mücke M, Mochamat CH, Peuckmann‐Post V, Minton O, Stone P, Radbruch L. Pharmacological treatments for fatigue associated with palliative care: executive summary of a Cochrane Collaboration systematic review. J Cachexia Sarcopenia Muscle 2016;7:23–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Anker SD, Coats AJ, Morley JE. Evidence for partial pharmaceutical reversal of the cancer anorexia‐cachexia syndrome: the case of anamorelin. J Cachexia Sarcopenia Muscle 2015;6:275–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Fearon K, Argiles JM, Baracos VE, Bernabei R, Coats A, Crawford J, Deutz NE, Doehner W, Evans WJ, Ferrucci L, Garcia JM, Gralla RJ, Jatoi A, Kalantar‐Zadeh K, Lainscak M, Morley JE, Muscaritoli M, Polkey MI, Rosano G, Rossi‐Fanelli F, Schols AM, Strasser F, Vellas B, von Haehling S, Anker SD. Request for regulatory guidance for cancer cachexia intervention trials. J Cachexia Sarcopenia Muscle 2015;6:272–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Rutten IJ, van Dijk DP, Kruitwagen RF, Beets‐Tan RG, Olde Damink SW, van Gorp T. Loss of skeletal muscle during neoadjuvant chemotherapy is related to decreased survival in ovarian cancer patients. J Cachexia Sarcopenia Muscle 2016;7:458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Penna F, Bonetto A, Aversa Z, Minero VG, Rossi Fanelli F, Costelli P, Muscaritoli M. Effect of the specific proteasome inhibitor bortezomib on cancer‐related muscle wasting. J Cachexia Sarcopenia Muscle 2016;7:345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Toledo M, Penna F, Oliva F, Luque M, Betancourt A, Marmonti E, López‐Soriano FJ, Argilés JM, Busquets S. A multifactorial anti‐cachectic approach for cancer cachexia in a rat model undergoing chemotherapy. J Cachexia Sarcopenia Muscle 2016;7:48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Musolino V, Palus S, Tschirner A, Drescher C, Gliozzi M, Carresi C, Vitale C, Muscoli C, Doehner W, von Haehling S, Anker SD, Mollace V, Springer J. Megestrol acetate improves cardiac function in a model of cancer cachexia‐induced cardiomyopathy by autophagic modulation. J Cachexia Sarcopenia Muscle 2016;7:555–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Chen JA, Splenser A, Guillory B, Luo J, Mendiratta M, Belinova B, Halder T, Zhang G, Li YP, Garcia JM. Ghrelin prevents tumour‐ and cisplatin‐induced muscle wasting: characterization of multiple mechanisms involved. J Cachexia Sarcopenia Muscle 2015;6:132–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Giles K, Guan C, Jagoe TR, Mazurak V. Diet composition as a source of variation in experimental animal models of cancer cachexia. J Cachexia Sarcopenia Muscle 2016;7:110–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Dwarkasing JT, Boekschoten MV, Argilès JM, van Dijk M, Busquets S, Penna F, Toledo M, Laviano A, Witkamp RF, van Norren K. Differences in food intake of tumour‐bearing cachectic mice are associated with hypothalamic serotonin signalling. J Cachexia Sarcopenia Muscle 2015;6:84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Toneto AT, Ferreira Ramos LA, Salomão EM, Tomasin R, Aereas MA, Gomes‐Marcondes MC. Nutritional leucine supplementation attenuates cardiac failure in tumour‐bearing cachectic animals. J Cachexia Sarcopenia Muscle 2016;7:577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Gu W, Zhang G, Sun L, Ma Q, Cheng Y, Zhang H, Shi G, Zhu Y, Ye D. Nutritional screening is strongly associated with overall survival in patients treated with targeted agents for metastatic renal cell carcinoma. J Cachexia Sarcopenia Muscle 2015;6:222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wakabayashi H, Matsushima M, Uwano R, Watanabe N, Oritsu H, Shimizu Y. Skeletal muscle mass is associated with severe dysphagia in cancer patients. J Cachexia Sarcopenia Muscle 2015;6:351–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Saitoh M, Ishida J, Konishi M, Springer J. The concept that focuses on oral motor and feeding function in cancer patients with muscle wasting: skeletal muscle mass is associated with severe dysphagia in cancer patients. J Cachexia Sarcopenia Muscle 2016;7:233–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Amano K, Maeda I, Morita T, Okajima Y, Hama T, Aoyama M, Kizawa Y, Tsuneto S, Shima Y, Miyashita M. Eating‐related distress and need for nutritional support of families of advanced cancer patients: a nationwide survey of bereaved family members. J Cachexia Sarcopenia Muscle 2016;7:527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. von Haehling S, Morley JE, Coats AJ, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2015. J Cachexia Sarcopenia Muscle 2015;6:315–316. [DOI] [PMC free article] [PubMed] [Google Scholar]


