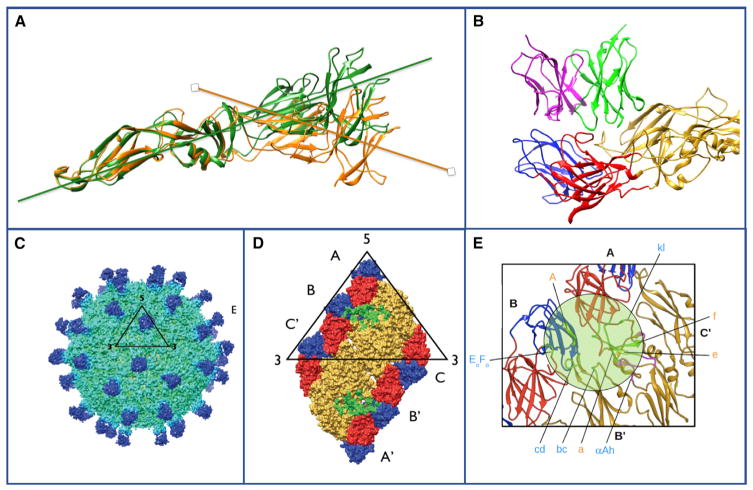
Figure 2. Quaternary Epitopes on Dengue Virus Particles.
(A) The domain I/II hinge angle in soluble recombinant dengue E protein differs from that in the virus. The structure of the human dengue serotype protein has been determined by crystallography (green, based on PDB: 10AN) or by cryo-EM (orange, based on EMDB: 2442). In the particle, the E protein is maintained with a “bent” conformation at the inter-domain hinge. In the crystal structure, the soluble protein achieves a more linear orientation (green).
(B) The human mAb 2D22 that is specific for serotype 2 dengue viruses engages a broad surface on the envelope protein, engaging multiple viral protein domains. The Fab is on top, with heavy chain in green and light chain in pink; the dengue E protein is on bottom with domain I in red, domain II in yellow, and domain III in blue. Based on PDB: 4UIF.
(C) Dengue virus 3-specific human mAb 5J7 recognizes a very complex quaternary epitope. The 9Å cryo-EM map of DENV3 complexed with Fab 5J7. The black triangle and numbers represent an icosahedral asymmetric unit and the icosahedral vertices, respectively.
(D) The epitope recognized by HMAb 5J7. One molecule of Fab 5J7 binds to three E proteins. Top left panel shows a raft of E proteins (containing two asymmetric units). The three individual E protein molecules in an asymmetric unit are labeled as A, B, and C, and the corresponding E proteins in the neighboring asymmetric unit as A’, B’, and C’. DI, DII, and DIII are colored in red, yellow, and blue, respectively.
(E) Enlarged view of the 5J7 epitope. The residues on the E protein that interact with heavy or light chains of the Fab are shown as green or magenta spheres, respectively. Based on PDB: 3J6U, the cryo-EM structure of dengue virus serotype 3 in complex with human antibody 5J7 Fab. All structures were determined in collaboration with the laboratory of Sheemei Lok.