Figure 3. Schematics of cell type-specific RNA aptamers used as delivery agents.
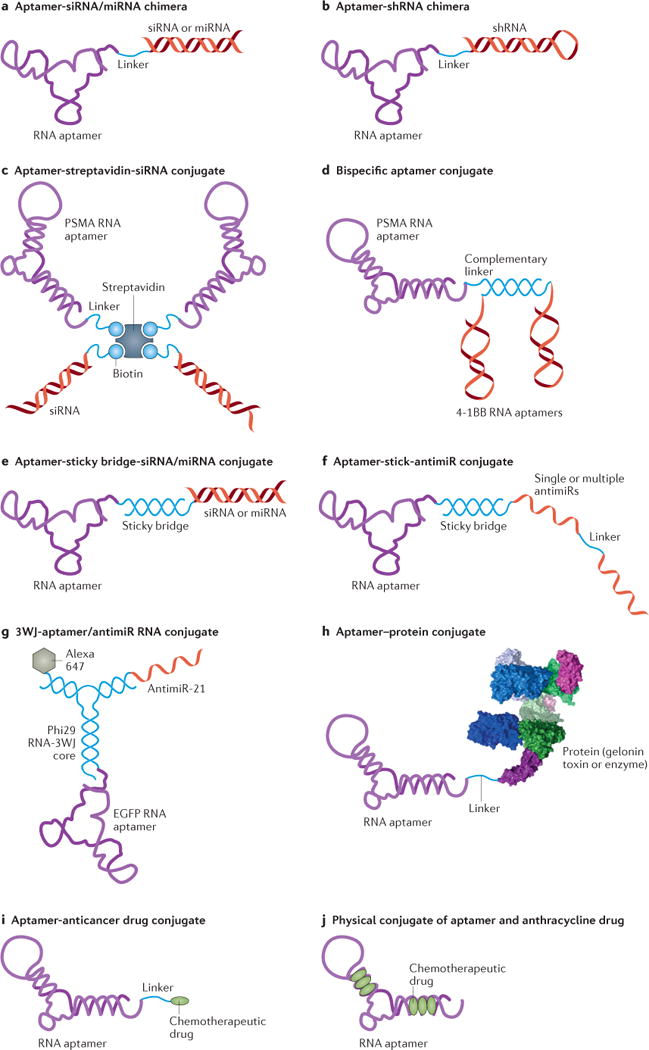
A) An aptamer-siRNA/miRNA chimera. The chimera is synthesized as two pieces followed by an annealing step to make the chimeric RNA molecule. B) An aptamer-shRNA chimera synthesized as one piece. C) An aptamer-streptavidin-siRNA conjugate. The siRNA and PSMA RNA aptamers are chemically conjugated with a biotin group. Then the two biotinylated siRNAs and two aptamers are non-covalently assembled via a streptavidin connector. D) The bispecific PSMA-4-1BB aptamer conjugate. The PSMA RNA aptamer and a bivalent 4-1BB RNA aptamer are tethered to complementary linker sequences and hybridized through Watson-Crick base pairing. E) An aptamer-sticky bridge-siRNA/miRNA conjugate. The aptamer and siRNA/miRNA are appended to complementary GC-rich bridge sequences and annealed by simple mixing that allows Watson-Crick base pairing. F) An aptamer-sticky bridge-antimiR conjugate. The single or multiple antimiR oligonucleotides are hybridized with the aptamers via a GC-rich sticky bridge. G) The 3WJ-aptamer/antimiR RNA conjugate. It contains an epidermal growth factor receptor (EGFR) aptamer as a targeting agent, an anti-miR-21 sequence as a therapeutic agent, a fluorescent dye (Alexa647) as an imaging agent, and a three-way junction (3WJ) motif as a molecular scaffold. H) An aptamer-protein conjugate. Chemical synthesis incorporates a primary amino group at the 5′-end of the PSMA aptamer, allowing chemical modification with a cross-linker agent (SPDP, N-Succinimidyl 3-[2-pyridyldithio]-propionate), and subsequent conjugation with the cysteine residue of gelonin toxin through a disulfide linkage. I) An aptamer-anticancer drug conjugate. It is made by using an acid-labile acylhydrazone linkage or formaldehyde linkage. J) Physical conjugation between an aptamer and anthracycline drug (dox) through intercalation.
