Fig. 2.
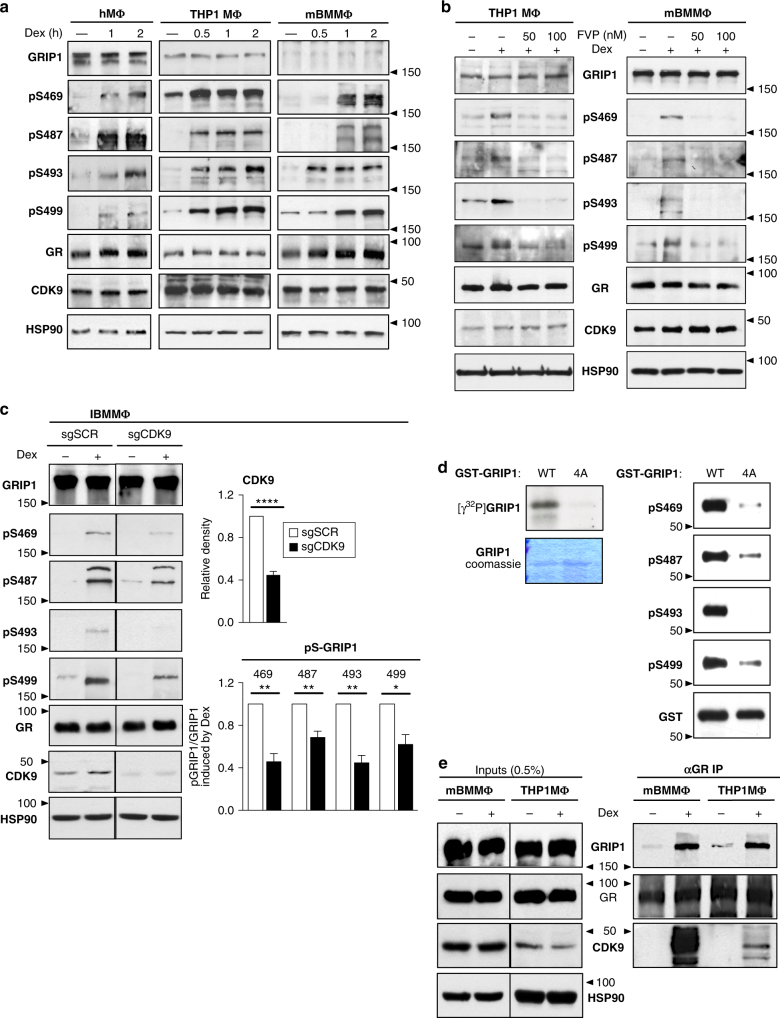
GRIP1 is a substrate for dexamethasone (Dex)-induced phosphorylation by CDK9 in macrophages. a hMΦ, THP1 cells or BMMΦ were treated with Dex for the times indicated and the levels of GRIP1 (total or phosphorylated (p) at S469, S487, S493, and S499), GR, CDK9 and HSP90 as a loading control were assessed by immunoblotting (quantified in Supplementary Fig. 5a). b THP1 cells or mBMMΦ were treated with Dex±flavopiridol (FVP) at indicated concentrations for 2 h and GRIP1 phosphorylation was visualized by immunoblotting (quantified in Supplementary Fig. 5b). c Immortalized BMMΦ were depleted of CDK9 (single guide (sg)CDK9 vs. scrambled sgSCR control) as described in Methods and treated ±Dex for 1 h. The levels of GRIP1, phospho-(p)GRIP1, GR, CDK9 and HSP90 were assayed by western blot and quantified by densitometry; CDK9 levels or ratios of pGRIP1/GRIP1 in sgCDK9 cells are expressed relative to those in sgSCR cells (=1). Samples were compared by unpaired, two-tailed Student’s t-test; means + standard error of the mean (SEM) are shown (n ≥ 3, *P < 0.05, **P < 0.01). d E. coli-produced affinity-purified GST–GRIP1322-631 WT and S469A/S487A/S493A/S499A (4A) were subjected to in vitro kinase assays with baculovirus-expressed cyclin T1-CDK9. GRIP1 phosphorylation was assessed by autoradiography (left) and immunoblotting (right) with Coomassie blue staining (left) or immunoblotting for GST-tag (right) showing equal loading. e BMMΦ or THP1 cells were treated ±Dex for 1 h, double cross-linked and GR immunoprecipitations (IP) performed (see “Methods”). Inputs and IPs were immunoblotted for GRIP1, GR and CDK9 (with HSP90 as an input loading control). Full-size western blots are shown in Supplementary Fig. 10a–e