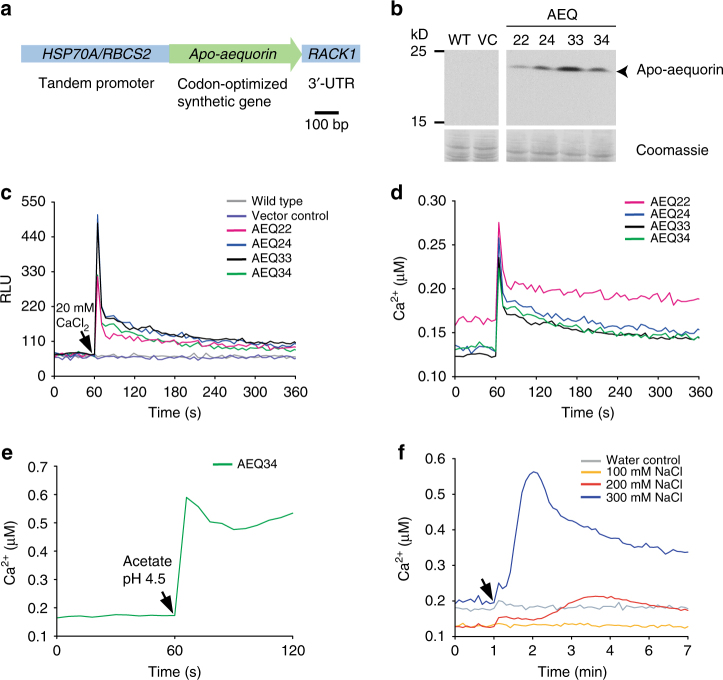
Fig. 3.
Establishment of an aequorin-based calcium assay in C. reinhardtii and its applicability for abiotic stresses. a The apo-aequorin expression cassette includes a codon optimized gene and cis-acting elements for efficient expression (Supplementary Fig. 7). b Immunoblot showing the expression of apo-aequorin (22 kD) in transgenic lines AEQ22, AEQ24, AEQ33, and AEQ34 compared to wild type (WT) and a vector control (VC). As a loading control, selected protein bands from the Coomassie-stained PVDF membrane are shown. Another biological replicate showed qualitatively identical results. c, d External calcium triggers luminescence in aequorin-expressing lines. After measuring the resting Ca2+ concentration in relative light units (RLU), 20 mM of external CaCl2 were added as indicated by the black arrowhead. Obtained RLU values (c) were converted to relative Ca2+ concentrations (d) by triggering maximal luminescence (Supplementary Fig. 8). e Acidification of the medium causes a prompt increase in cytosolic Ca2+ ions. After measurement of the resting Ca2+ concentration, sodium acetate buffer pH 4.5 was added to a final concentration of 20 mM (black arrowhead) to AEQ34 cells in HEPES buffer pH 7.4. f Salt stress results in an increase in Ca2+ ions. Coelenterazine loaded cells of transgenic aequorin reporter line AEQ34 were treated with increasing concentrations of NaCl (black arrowhead) as indicated. As a control, an equivalent volume of deionized water was used. c–f Each line in the graphs represents the mean of three biological replicates and each biological replicate includes three technical replicates. All experiments were replicated twice except for e, which was done once