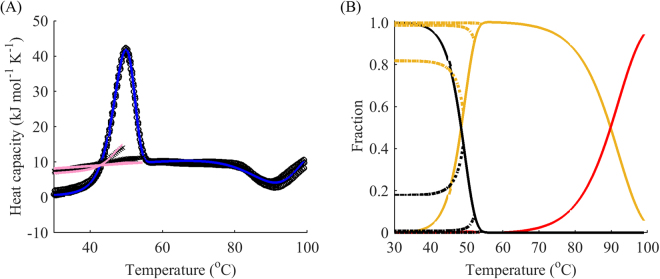
Figure 10.
One-step irreversible unfolding of LinB with one additional exothermal peak at high temperatures. (A) DSC data (black) for the denaturation of LinB: first runs (circles), reheated runs for terminal temperatures 49, 52 and 54 °C (crosses), fitted curves for the first run (blue) and reheated runs (pink) from model B plus one negative peak at high temperatures. (B) Respective modelled fractions of states for a given temperature: native folded (black), intermediate (yellow) and denatured (red) states, cooling for all the states is showed by dotted lines. The scan rate was 1 °C min−1. Cooling from the peak temperature resulted in less than 20% of the protein in the native state.