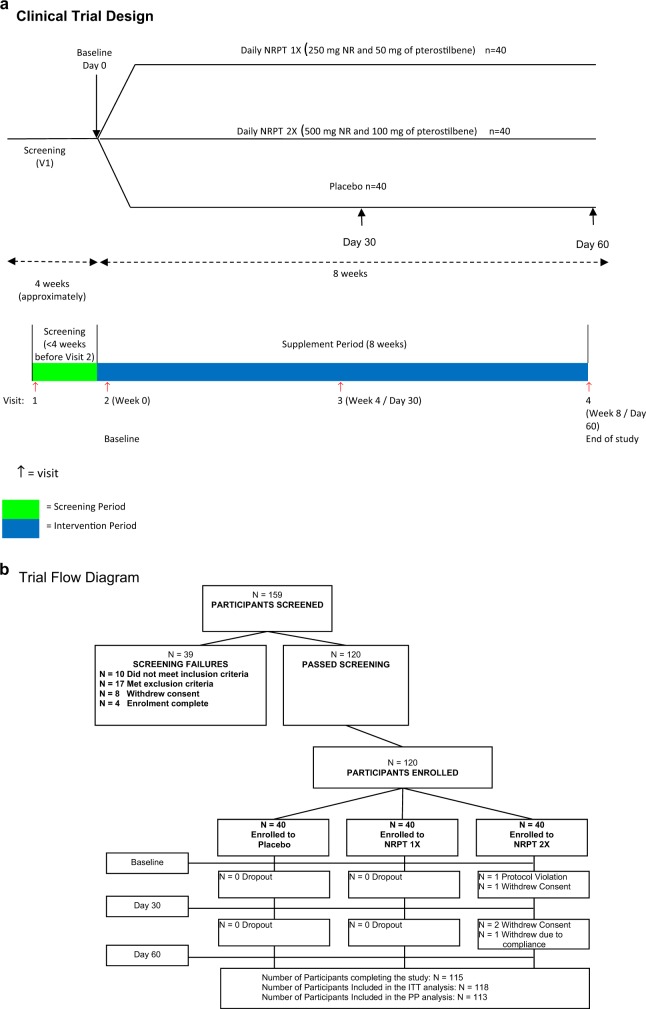
Fig. 1.
Clinical trial diagrams. a Clinical Trial Design diagram. Schematic depicting the randomized, double-blind, placebo controlled, three-arm parallel group study. The study consisted of a single eight-week study period. Clinic visits occurred at day 0 (baseline), day 30, and day 60. Subjects were asked to fast 12 h prior to each clinic visit. Each clinic visit consisted of a physical exam including as well as blood draws to evaluate safety and efficacy endpoints of the trial. b Clinical Trial Flow Diagram. Schematic depicting recruitment and disposition of study participants. A total of 159 potential subjects were screened to successfully enroll 120 eligible subjects and randomize them 1:1:1 to the three arms. One-hundred fifteen subjects completed the 60-day study