Abstract
Introduction
Polymorphisms of the vitamin D receptor (VDR) gene have been investigated in various case-control studies to evaluate prostate cancer susceptibility; however, published data on the association between vitamin D receptor gene FokI polymorphism and prostate cancer risk are inconclusive.
Material and methods
To assess the impact of vitamin D receptor gene FokI polymorphism, we performed a meta-analysis of eligible studies including 9,720 patients and 9,710 control subjects.
Results
The overall results indicated no obvious association of this variant on prostate cancer risk. However, in subgroup analysis by ethnicity, positive associations existed in Caucasian descendents for allelic contrast (OR = 1.03, 95% CI: 1.00–1.06, pheterogeneity = 0.552, p = 0.026) and the dominant genetic model (OR = 1.03, 95% CI: 1.00–1.05, pheterogeneity = 0.856, p = 0.032). In the subgroup analysis by tumor stage, there was a significant association between this variant and advanced prostate cancer under the recessive genetic model (OR = 1.15, 95% CI: 1.01–1.32, pheterogeneity = 0.469, p = 0.032). In the subgroup analysis by source of control, association of the VDR FokI polymorphism and prostate cancer susceptibility was also found in population-based studies under homozygote comparison and the recessive genetic model.
Conclusions
The VDR FokI polymorphism may contribute to the risk of developing prostate cancer in Caucasian and population-based studies. Further large, well-designed studies are warranted to confirm this conclusion in more detail.
Keywords: vitamin D receptor, polymorphism, cancer risk
Introduction
Prostate cancer (PCa) is one of the most commonly diagnosed solid neoplasms in the male population [1]. In the United States, prostate cancer is the second leading cause of carcinoma deaths among males, with an estimated 192,000 new cases and 27,000 deaths in 2009 [2]. In European countries, it is recognized as the most common solid tumor, with an incidence rate of 214 cases in every thousand men, outnumbering lung and colorectal cancer [3]. Although epidemiological research indicated that the incidence of prostate cancer in Asians is much lower than that in the USA and European countries, the incidence and mortality rate of this disease have rapidly increased among Chinese men [4, 5]. Data from the Global Estimates of Cancer indicated that the age standardized incidence rate of PCa in China was 4.3 per 10,000 males in 2008 [6]. With the improvement of diagnostic techniques and the aging of the population, morbidity and mortality of PCa show an increasing trend. So far, many factors including lifestyle, environment and race have been demonstrated as possible contributors to the risk of PCa [1]. However, the etiology of PCa remains unclear for the reason that a complex interplay between genetic and environmental factors is involved in the development and occurrence of PCa.
The human vitamin D receptor (VDR), located on chromosome 12q12–q14, is a nuclear receptor gene with 75 kb and consists of 11 exons and 11 introns [7]. VDR acts as a ligand-dependent transcriptional factor found in various types of tissues (including the prostate) by the interaction with vitamin D [8]. It has been found that 1,25-dihydroxyvitamin D3 [1,25(OH)2D3], the active form of vitamin D, could enhance immune regulation, promote cell differentiation and inhibit tumor invasion and metastasis via the biological effects mediated by VDR in the prostate [9–11]. Therefore, a less active VDR could be associated with either a more aggressive disease or increased susceptibility to cancer risk [12]. Studies have shown that several single-nucleotide polymorphisms (SNPs) of VDR, which potentially affect the receptor binding of 1,25(OH)2D3, may influence vitamin D biological activity and confer susceptibility to prostate cancer [13]. Among them, the most commonly studied SNP is the restriction fragment length polymorphism of FokI, which is detected by the endonuclease FokI. The FokI located on the coding region (exon 2) of the VDR gene results in production of a VDR protein three amino acids longer [14].
A number of epidemiological studies have been carried out to explore the association between VDR FokI polymorphism and prostate cancer risk. However, the results of these studies remain controversial rather than conclusive, possibly because of conflicting results from different case-control studies. In 2006, Berndt and his group [15] performed a meta-analysis and found no statistically significant association between the FokI polymorphism and PCa risk. Ever since, new studies have provided additional data correlating with the VDR variants. Therefore, in this meta-analysis, the most up-to-date accumulated data from all eligible studies published were utilized to obtain a summary result of the association between the VDR FokI polymorphism and PCa susceptibility [16–36].
Material and methods
Search strategy and identification of relevant studies
PubMed, Web of Science and Embase database searches were carried out using the following terms: ‘vitamin D receptor’ or ‘VDR’, ‘prostate cancer’ and ‘polymorphism’ or ‘variant’ (last search updated on August 25, 2015). References of the relevant paper and retrieved articles were also identified by a manual search. Eligible studies had to meet all the following inclusion criteria: (a) uses an unrelated case-control design; (b) includes available genotype frequencies; (c) research published in English; (d) provides sufficient data to calculate the odds ratio (OR) with 95% confidence interval (CI).
Data extraction and quality assessment
Data were collected on the genotype of VDR FokI according to prostate cancer. For each publication, the data extraction was carried out by two of the investigators independently according to the inclusion criteria above. Disagreement was to be resolved through a discussion between two authors. If they could not reach a consensus, the problem was to be discussed comprehensively by all investigators. Furthermore, eligible studies containing information about clinical stage of PCa were divided into two groups: localized and advanced (including cases with bone metastasis). Information from enrolled studies was extracted as below: first author’s name, year of publication, ethnicity of subjects, sources of controls, sample size in cases and controls, number of cases and controls with wild type and variant allele, and p-value for Hardy-Weinberg Equilibrium (HWE).
Statistical analysis
Odds ratios (ORs) with 95% CIs were used to assess the strength of association between the polymorphism in VDR FokI and prostate cancer risk. Five genetic contrasts were used to evaluate the association: allelic contrast (f allele vs. F allele), homozygote comparison (ff vs. FF), heterozygote comparison (Ff vs. FF), dominant genetic model (ff + Ff vs. FF) and recessive genetic model (ff vs. Ff + FF). Stratified analyses were performed by ethnicity and source of controls (hospital-based, population-based and benign prostatic hyperplasia (BPH) based). The pooled ORs for the risk were calculated using the random effects model and fixed effects model. Heterogeneity assumption was assessed by the χ2-based Q test among the studies. The data were evaluated using random-effects models (the DerSimonian and Laird method) [37] in the presence of heterogeneity (p < 0.05) and fixed-effects (the Mantel-Haenszel method) models [38] were performed in the absence of heterogeneity (p > 0.05). The HWE was calculated by the Pearson χ2 test for goodness of fit. The Z-test was performed to evaluate the statistical significance of the summary OR; a p-value of < 0.05 was considered significant. The statistic of I2 was also used to test the heterogeneity, with I2 > 75%, 25–75% and < 25% to represent high, moderate and low degree of inconsistency, respectively. Significance of the intercept was determined by the t-test as suggested by Egger (p < 0.01 represents a statistically significant publication bias) [39]. All the statistical analyses were conducted using STATA version 10.0 (Stata Corporation, College Station, TX).
Results
Study characteristics
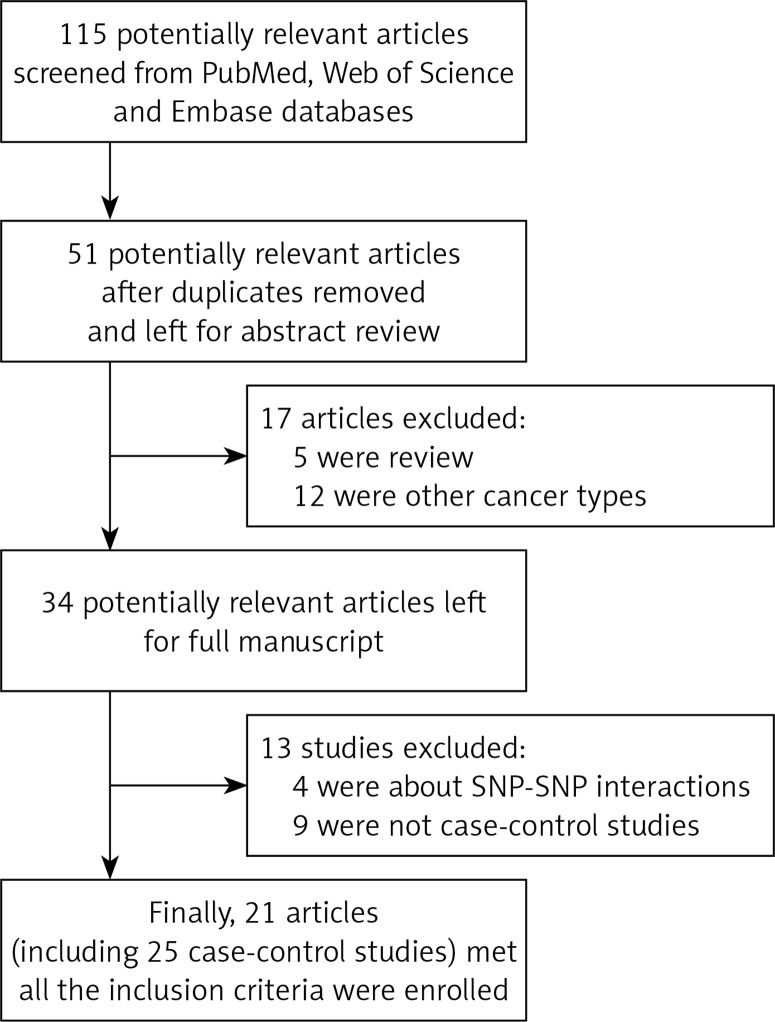
A total of 21 articles (including 25 case-control studies) that met all the inclusion criteria were enrolled in our study (Figure 1). Characteristics of the eligible studies are summarized in Table I. In general, 9,720 prostate cancer patients and 9,710 control subjects concerning the VDR FokI polymorphism were assessed. In the subgroup of ethnicity, 14 were carried out in Caucasian descendents, three were in Asian descendents, three studies were in Arabians and three in African-Americans. Only one article was in Spanish descendents. Hospital-based controls were carried out in 18 of these studies. The classical genotyping method called polymerase chain reaction-restriction fragment length polymorphism (RFLP) was used in 13 comparisons. Five studies performed TaqMan real-time polymerase chain reaction (PCR).
Figure 1.
Study flow chart for the process of selecting the enrolled studies
Table I.
Characteristics of studies of the vitamin D receptor (VDR) FokI gene polymorphism included in this meta-analysis
| First author | Year | Ethnicity | Source of control | Genotyping | Sample size of case | Sample size of control | PHWE | Frequencyof f allele | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ff | fF | FF | Total | ff | fF | FF | Total | |||||||
| Correa | 1999 | Caucasian | Population-based | PCR-RFLP | 10 | 58 | 50 | 118 | 9 | 42 | 38 | 89 | 0.598 | 0.337 |
| Chokkalingam | 2001 | Asian | Hospital-based | PCR-RFLP | 41 | 95 | 51 | 187 | 62 | 153 | 87 | 302 | 0.725 | 0.459 |
| Tayeb | 2004 | Caucasian | Population-based | PCR-RFLP | 2 | 10 | 16 | 28 | 11 | 24 | 21 | 56 | 0.391 | 0.411 |
| Oakley-Girvan | 2004 | Caucasian | Population-based | PCR-RFLP | 27 | 120 | 85 | 232 | 26 | 77 | 68 | 171 | 0.586 | 0.377 |
| Oakley-Girvan | 2004 | African-American | Population-based | PCR-RFLP | 4 | 25 | 84 | 113 | 5 | 42 | 74 | 121 | 0.752 | 0.215 |
| Bodiwala | 2004 | Caucasian | BPH-based | PCR-RFLP | 60 | 163 | 145 | 368 | 35 | 100 | 108 | 243 | 0.137 | 0.350 |
| Cheteri | 2004 | Caucasian | Population-based | PCR-RFLP | 101 | 247 | 204 | 552 | 80 | 234 | 207 | 521 | 0.305 | 0.378 |
| Mishra | 2005 | Arabian | Hospital-based | PCR-RFLP | 5 | 45 | 78 | 128 | 16 | 69 | 62 | 147 | 0.622 | 0.344 |
| John | 2005 | Caucasian | Population-based | PCR-RFLP | 69 | 203 | 153 | 425 | 57 | 209 | 171 | 437 | 0.581 | 0.370 |
| Hayes | 2005 | Caucasian | Population-based | DGGE | 112 | 359 | 340 | 811 | 98 | 322 | 293 | 713 | 0.526 | 0.363 |
| Cicek | 2006 | Mixed | Population-based | PCR-RFLP | 71 | 191 | 77 | 339 | 77 | 202 | 200 | 479 | 0.034 | 0.372 |
| Huang | 2006 | Asian | Hospital-based | PCR-RFLP | 87 | 204 | 125 | 416 | 119 | 248 | 135 | 502 | 0.806 | 0.484 |
| Rukin | 2007 | Caucasian | BPH-based | Pyrosequencing | 166 | 203 | 61 | 430 | 135 | 141 | 44 | 320 | 0.461 | 0.642 |
| Mikhak | 2007 | Caucasian | Population-based | Taqman | 101 | 337 | 232 | 670 | 108 | 310 | 255 | 673 | 0.398 | 0.391 |
| Li | 2007 | Caucasian | Population-based | PCR-RFLP | 152 | 475 | 383 | 1010 | 206 | 655 | 571 | 1432 | 0.413 | 0.373 |
| Holick | 2007 | Caucasian | Population-based | SNPlex assay | 100 | 261 | 222 | 583 | 85 | 245 | 222 | 552 | 0.204 | 0.376 |
| Torkko | 2008 | Spanish | Population-based | Taqman | 26 | 70 | 45 | 141 | 57 | 125 | 91 | 273 | 0.249 | 0.438 |
| Torkko | 2008 | Caucasian | Population-based | Taqman | 67 | 209 | 168 | 444 | 63 | 227 | 198 | 488 | 0.87 | 0.362 |
| Holt | 2009 | African-American | Population-based | SNPlex assay | 2 | 48 | 65 | 115 | 4 | 24 | 39 | 67 | 0.904 | 0.239 |
| Holt | 2009 | Caucasian | Population-based | SNPlex assay | 108 | 335 | 262 | 705 | 101 | 352 | 263 | 716 | 0.332 | 0.387 |
| Bai | 2009 | Asian | Hospital-based | PCR-RFLP | 26 | 63 | 33 | 122 | 37 | 55 | 38 | 130 | 0.08 | 0.496 |
| Rowland | 2013 | African-American | Population-based | Taqman | 24 | 161 | 236 | 421 | 10 | 90 | 134 | 234 | 0.287 | 0.235 |
| Rowland | 2013 | Caucasian | Population-based | Taqman | 193 | 598 | 406 | 1197 | 112 | 413 | 311 | 836 | 0.171 | 0.381 |
| Yousaf | 2014 | Arabian | Hospital-based | PCR-RFLP | 2 | 0 | 39 | 41 | 13 | 0 | 95 | 108 | 0 | 0.120 |
| Atoum | 2015 | Arabian | Population-based | PCR | 10 | 76 | 38 | 124 | 6 | 66 | 28 | 100 | 0 | 0.390 |
HWE – Hardy-Weinberg equilibrium of controls, RFLP – restriction fragment length polymorphism, BPH – benign prostatic hyperplasia.
Quantitative synthesis
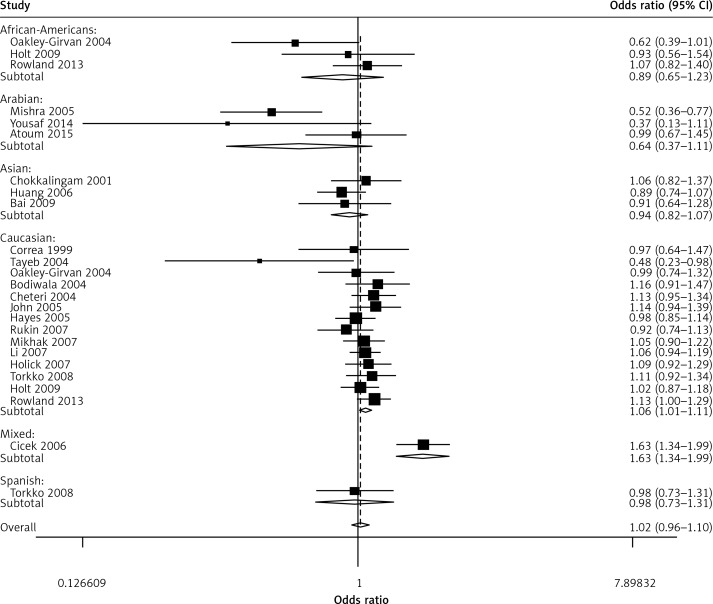
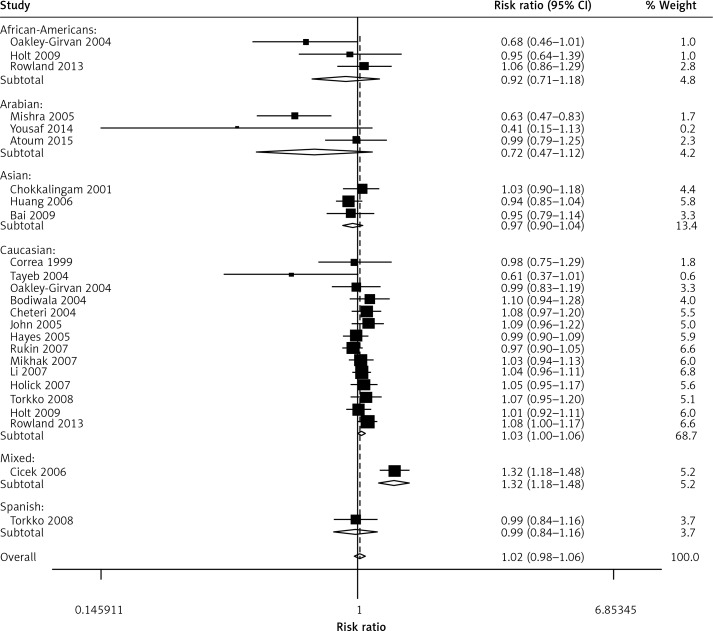
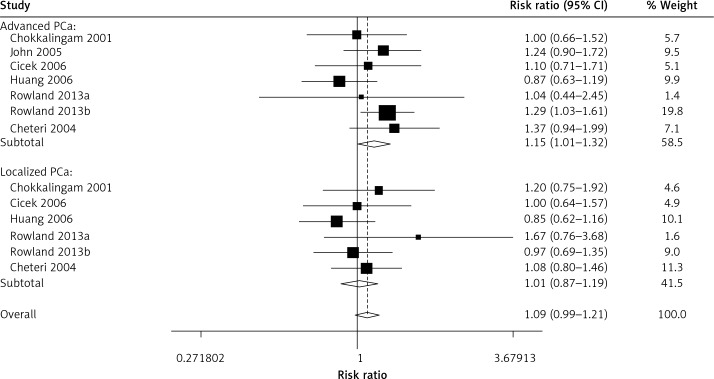
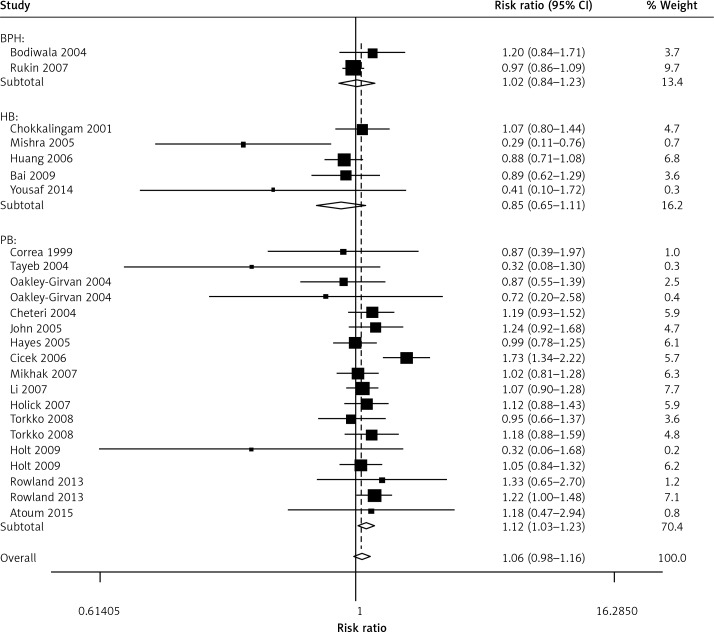
When all the eligible studies were pooled into the meta-analysis (Table II), no obvious association was observed in the overall analysis between prostate cancer risk and the VDR FokI variant genotypes: allelic comparison (random-effects OR = 1.02, 95% CI: 0.96–1.10, pheterogeneity < 0.001, p = 0.498, I2 = 56.6) (Figure 2), homozygote comparison (random-effects OR = 1.08, 95% CI: 0.95–1.03, pheterogeneity = 0.020, p = 0.216, I2 = 40.3), heterozygote comparison (random-effects OR = 1.06, 95% CI: 0.97–1.17, pheterogeneity = 0.002, p = 0.211, I2 = 50.7), dominant genetic model (random-effects OR = 1.05, 95% CI: 0.95–1.16, pheterogeneity < 0.001, p = 0.326, I2 = 57.0) and the recessive genetic model (fixed-effects OR = 1.03, 95% CI: 0.96–1.11, pheterogeneity = 0.280, p = 0.406, I2 = 12.8). However, in the subgroup analysis by ethnicity, positive associations between VDR FokI polymorphism and prostate cancer risk were found in Caucasian descendents for allelic contrast (fixed-OR = 1.03, 95% CI: 1.00–1.06, Pheterogeneity = 0.552, p = 0.026, I2 = 0) (Figure 3) and the dominant genetic model (fixed-effects OR = 1.03, 95% CI: 1.00–1.05, pheterogeneity = 0.856, p = 0.032, I2 = 0), but not in Asian descendents (allelic comparison fixed-effects OR = 0.97, 95% CI: 0.90–1.04, pheterogeneity = 0.536, p = 0.352, I2 = 0), Arabians (allelic comparison random-effects OR = 0.64, 95% CI: 0.37–1.11, pheterogeneity = 0.036, p = 0.114, I2 = 69.8) or African-Americans (allelic comparison fixed-effects OR = 0.92, 95% CI: 0.71–1.18, pheterogeneity = 0.151, p = 0.500, I2 = 47.2). In the subgroup analysis by tumor stage, there was a significant association between this variant and advanced prostate cancer under the recessive genetic model (fixed-effects OR = 1.15, 95% CI: 1.01–1.32, pheterogeneity = 0.469, p = 0.032, I2 = 0) (Figure 4). Furthermore, a significant association between the VDR FokI polymorphism and prostate cancer was also found in population-based studies under homozygote comparison (random-effects OR = 1.12, 95% CI: 1.03–1.23, pheterogeneity = 0.143, p = 0.010, I2 = 26.7) (Figure 5) and the recessive genetic model (fixed-effects OR = 1.09, 95% CI: 1.01–1.17, pheterogeneity = 0.669, p = 0.034, I2 = 0), with no association among hospital-based studies (homozygote comparison: fixed-effects OR = 0.85, 95% CI: 0.65–1.11, pheterogeneity = 0.090, p = 0.223, I2 = 50.3; recessive genetic model: fixed-effects OR = 0.83, 95% CI: 0.64–1.07, pheterogeneity = 0.194, p = 0.155, I2 = 34.1) and BPH based studies in homozygote comparison (fixed-effects OR = 1.02, 95% CI: 0.84–1.23, pheterogeneity = 0.226, p = 0.869, I2 = 31.9) and the recessive genetic model (fixed-effects OR = 0.95, 95% CI: 0.81–1.11, pheterogeneity = 0.317, p = 0.522, I2 = 0.1).
Table II.
Stratified analyses of the vitamin D receptor gene FokI polymorphism and prostate cancer risk
| Variables | Na | Cases/controls | f allele vs. F allele | ff vs. FF | ff + Ff vs. FF | ff vs. Ff + FF | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | Pb | P | I2 | OR (95% CI) | Pb | P | I2 | OR (95% CI) | Pb | P | I2 | OR (95% CI) | Pb | P | I2 | |||
| Total | 25 | 9720/9710 | 1.02 (0.96–1.10) | < 0.001 | 0.498 | 56.6 | 1.08 (0.95–1.03) | 0.020 | 0.216 | 40.3 | 1.05 (0.95–1.16) | < 0.001 | 0.326 | 57.0 | 1.03 (0.96–1.11) | 0.280c | 0.406 | 12.8 |
| Ethnicity: | ||||||||||||||||||
| Caucasian | 14 | 7573/7247 | 1.03 (1.00–1.06) | 0.552c | 0.026 | 0 | 1.06 (1.00–1.13) | 0.544c | 0.065 | 0 | 1.03 (1.00–1.05) | 0.856c | 0.032 | 0 | 1.05 (0.98–1.13) | 0.571c | 0.183 | 0 |
| Asian | 3 | 725/934 | 0.97 (0.90–1.04) | 0.536c | 0.352 | 0 | 0.93 (0.80–1.08) | 0.540c | 0.344 | 0 | 0.99 (0.93–1.05) | 0.548c | 0.668 | 0 | 0.90 (0.75–1.08) | 0.444c | 0.271 | 0 |
| Arabian | 3 | 293/355 | 0.64 (0.37–1.11) | 0.036 | 0.114 | 69.8 | 0.54 (0.21–1.39) | 0.101c | 0.202 | 56.4 | 0.78 (0.55–1.13) | 0.029 | 0.189 | 71.7 | 0.61 (0.25–1.50) | 0.138c | 0.279 | 49.6 |
| African-American | 3 | 649/422 | 0.92 (0.71–1.18) | 0.151c | 0.50 | 47.2 | 0.89 (0.42–1.86) | 0.261c | 0.749 | 25.6 | 0.92 (0.71–1.19) | 0.112c | 0.531 | 54.4 | 0.91 (0.42–1.95) | 0.251c | 0.801 | 27.7 |
| Spanish | 1 | 141/273 | 0.98 (0.73–1.31) | – | 0.888 | – | 0.92 (0.51–1.16) | – | 0.787 | – | 1.07 (0.69–1.65) | – | 0.771 | – | 0.86 (0.51–1.44) | – | 0.557 | – |
| Mixed | 1 | 339/479 | 1.64 (1.34–1.99) | – | < 0.001 | – | 2.40 (1.58–3.63) | – | < 0.001 | – | 2.44 (1.78–3.33) | – | < 0.001 | – | 1.38 (0.97–1.98) | – | 0.075 | – |
| Source of control: | ||||||||||||||||||
| Population-based | 17 | 8028/7958 | 1.03 (1.00–1.07) | 0.017c | 0.051 | 46.3 | 1.12 (1.03–1.23) | 0.143c | 0.010 | 26.7 | 1.10 (0.99–1.22) | 0.002 | 0.090 | 56.0 | 1.09 (1.01–1.17) | 0.669c | 0.034 | 0 |
| Hospital-based | 5 | 894/1189 | 0.81 (0.63–1.04) | 0.022 | 0.100 | 65.1 | 0.85 (0.65–1.11) | 0.090c | 0.223 | 50.3 | 0.94 (0.83–1.06) | 0.026 | 0.311 | 63.9 | 0.83 (0.64–1.07) | 0.194c | 0.155 | 34.1 |
| BPH | 2 | 798/563 | 1.02 (0.90–1.15) | 0.133c | 0.811 | 55.7 | 1.02 (0.84–1.23) | 0.226c | 0.869 | 31.9 | 1.02 (0.93–1.13) | 0.171c | 0.629 | 46.8 | 0.95 (0.81–1.11) | 0.317c | 0.522 | 0.1 |
Number of comparisons
p-value of Q-test for heterogeneity test (Pheter).
A random-effects model was performed when P value for heterogeneity test < 0.05; otherwise, a fixed-effects model was used.
Figure 2.
Forest plot of prostate cancer risk associated with the VDR FokI gene polymorphism (allelic contrast of f allele vs. F allele, random-effects)
Figure 3.
Forest plot of prostate cancer risk associated with the VDR FokI gene polymorphism (allelic contrast of f allele vs. F allele) in the analyses stratified by ethnicity. The squares and horizontal lines represent the study-specific OR and 95% CI. The area of the squares reflects the weight (inverse of the variance). The diamond corresponds to the summary OR and 95% CI. Separate details are summarized in Table I
Figure 4.
Association between the VDR FokI gene polymorphism and different stages of prostate cancer (PCa), evaluated under the recessive genetic model. The area of the squares reflects the weight. The squares and horizontal lines represent the study-specific OR and 95% CI. The diamond corresponds to the summary OR and 95% CI
Figure 5.
Association between the VDR FokI gene polymorphism and prostate cancer (PCa) in subgroup analysis by source of control (under homozygote comparison). The area of the squares reflects the weight. The squares and horizontal lines represent the study-specific OR and 95% CI. The diamond corresponds to the summary OR and 95% CI
Publication bias
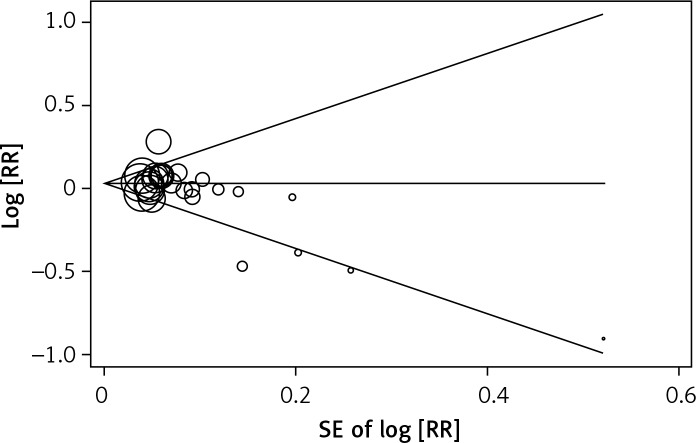
Egger’s test and Begg’s funnel plot were carried out to evaluate the literature’s publication bias. No obvious evidence of publication bias was found (f allele vs. F allele, t = –2.30, p = 0.031, Figure 6; ff vs. FF, t = –1.29, p = 0.209; ff vs. FF, t = –1.18, p = 0.251; ff + Ff vs. FF, t = –1.60, p = 0.251; ff vs. Ff + FF, t = –2.06, p = 0.051).
Figure 6.
Begg’s funnel plots to examine publication bias for allelic comparisons of VDR FokI polymorphism (f allele vs. F allele)
Discussion
Genetic susceptibility of solid tumors has led to growing attention to polymorphism studies of genes involved in the pathogenesis of carcinogenesis. Accumulating data have provided evidence that a low level of vitamin D is a risk factor for prostate cancer [40–42]. The vitamin D receptor, a significant regulator of the vitamin D pathway, could regulate conversion of serum 25(OH)D into the active hormone 1,25-dihydroxyvitamin D and mediate downstream transcription of a series of target genes [43]. Thus the FokI polymorphism of the VDR gene, encoding key proteins in vitamin D metabolism, has been chosen as a candidate polymorphism for prostate cancer susceptibility [44]. Nowadays, there is an increasing number of studies evaluating the FokI polymorphic variants of the VDR gene in prostate cancer susceptibility [45, 46]. Nevertheless, the association between this polymorphism and prostate cancer risk remains conflicting. Meta-analysis is used to combine the previous results to yield the most reliable and comprehensive conclusion because the individual research was too minor to achieve a valid conclusion [47, 48]. In this article, novel case-control studies from the last years were enrolled and a meta-analysis containing 9,720 prostate cancer patients and 9,710 controls from 25 independent case-control studies was performed.
Ethnicity is a significant biological factor which may affect the VDR functions via gene-gene interactions. When all the eligible studies were pooled into the meta-analysis, no obvious association of the VDR FokI polymorphism and prostate cancer risk was identified. However, in subgroup analysis by ethnicity, positive associations existed in Caucasian descendents (under the allelic contrast and dominant genetic model) but not in Asians, Arabians and African-Americans. Furthermore, in the analysis stratified by source of control, this VDR FokI variant was observed to increase the prostate cancer susceptibility in population-based studies (in homozygote comparison and the recessive genetic model), while no positive association was found in hospital-based and BPH-based controls. Nevertheless, some limitations of this pooled analysis ought to be addressed. The first limitation is the insufficient number of cases when specifying various ethnic backgrounds of prostate cancer. Only three studies were based on Asian [19, 34, 35] and Arabian descendents [16, 17, 28]. Second, some risk factors including age, smoking exposure, drinking, and family history were absent in this study. We tried to evaluate the gene and environment interaction effect on the susceptibility to prostate cancer. Unfortunately, the available data were not sufficient. Third, it has been suggested that positive results tend to be published faster than those with a ‘negative’ conclusion, which may need longer time to be accepted in time-lag bias [49]. In addition, combined interaction of multiple gene polymorphisms may have a stronger association with prostate cancer susceptibility, which is beyond the detection capacity of the present analysis.
On the other hand, the present meta-analysis has some key advantages compared with individual case-control studies. First, substantial numbers of cases and control subjects were enrolled from a variety of studies, which can significantly enhance the statistical power. Second, the quality of the case-control studies in this analysis was satisfactory and met the selection criteria with a wide representation. Third, no significant publication bias was detected through the qualitative funnel plot, which indicated that the conclusions are relatively stable and publication bias may not lead to an influence on the results of the present meta-analysis. In addition, studies using males with benign prostatic hyperplasia (BPH) as control subjects were enrolled. The reason for this inclusion was based on the assumption that BPH is a benign disease which has a similar probability for developing prostate cancer compared with normal prostate tissues. Previous epidemiological studies have provided no evidence concerning the association of increased BPH risk with VDR polymorphisms [50, 51]. Furthermore, the genotype distribution of the control population met the Hardy–Weinberg equilibrium in 23 of the studies.
In conclusion, this meta-analysis showed evidence that the VDR FokI variant may contribute to the risk for developing prostate cancer in a Caucasian population, but not with other descendents. Furthermore, there is a significant association between this variant and advanced prostate cancer. However, no association was detected in the overall analysis when all eligible studies were pooled into the analysis. Therefore, large, well-designed epidemiological studies, particularly referring to gene-environment interactions, are necessary to achieve a comprehensive conclusion of the association between VDR FokI polymorphism and prostate cancer risk.
Acknowledgments
Yuan-Yuan Mi, Yang-Zhi Chen and Jing Chen contributed equally to this work.
This study was supported by the foundation of High-Level Medical Talents Training Project (No. 2016CZBJ035).
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Heidenreich A, Bastian PJ, Bellmunt J, et al. EAU guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol. 2014;65:124–37. doi: 10.1016/j.eururo.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 3.Boyle P, Ferlay J. Cancer incidence and mortality in Europe, 2004. Ann Oncol. 2005;16:481–8. doi: 10.1093/annonc/mdi098. [DOI] [PubMed] [Google Scholar]
- 4.Quinn M, Babb P. Patterns and trends in prostate cancer incidence, survival, prevalence and mortality. Part I: international comparisons. BJU Int. 2002;90:162–73. doi: 10.1046/j.1464-410x.2002.2822.x. [DOI] [PubMed] [Google Scholar]
- 5.Ostrander EA, Stanford JL. Genetics of prostate cancer: too many loci, too few genes. Am J Hum Genet. 2000;67:1367–75. doi: 10.1086/316916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 7.Zmuda JM, Cauley JA, Ferrell RE. Molecular epidemiology of vitamin D receptor gene variants. Epidemiol Rev. 2000;22:203–17. doi: 10.1093/oxfordjournals.epirev.a018033. [DOI] [PubMed] [Google Scholar]
- 8.Miller GJ, Stapleton GE, Ferrara JA, et al. The human prostatic carcinoma cell line LNCaP expresses biologically active, specific receptors for 1 alpha,25-dihydroxyvitamin D3. Cancer Res. 1992;52:515–20. [PubMed] [Google Scholar]
- 9.Schwartz GG. Circulating vitamin D and risk of prostate cancer: letter. Cancer Epidemiol Biomarkers Prev. 2012;21:246. doi: 10.1158/1055-9965.EPI-11-0910. [DOI] [PubMed] [Google Scholar]
- 10.Bao BY, Yeh SD, Lee YF. 1alpha,25-dihydroxyvitamin D3 inhibits prostate cancer cell invasion via modulation of selective proteases. Carcinogenesis. 2006;27:32–42. doi: 10.1093/carcin/bgi170. [DOI] [PubMed] [Google Scholar]
- 11.Krishnan AV, Peehl DM, Feldman D. Inhibition of prostate cancer growth by vitamin D: regulation of target gene expression. J Cell Biochem. 2003;88:363–71. doi: 10.1002/jcb.10334. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz GG, Hulka BS. Is vitamin D deficiency a risk factor for prostate cancer? (Hypothesis) Anticancer Res. 1990;10:1307–11. [PubMed] [Google Scholar]
- 13.Raimondi S, Johansson H, Maisonneuve P, Gandini S. Review and meta-analysis on vitamin D receptor polymorphisms and cancer risk. Carcinogenesis. 2009;30:1170–80. doi: 10.1093/carcin/bgp103. [DOI] [PubMed] [Google Scholar]
- 14.Whitfield GK, Remus LS, Jurutka PW, et al. Functionally relevant polymorphisms in the human nuclear vitamin D receptor gene. Mol Cell Endocrinol. 2001;177:145–59. doi: 10.1016/s0303-7207(01)00406-3. [DOI] [PubMed] [Google Scholar]
- 15.Berndt SI, Dodson JL, Huang WY, Nicodemus KK. A systematic review of vitamin D receptor gene polymorphisms and prostate cancer risk. J Urol. 2006;175:1613–23. doi: 10.1016/S0022-5347(05)00958-4. [DOI] [PubMed] [Google Scholar]
- 16.Atoum MF, AlKateeb D, AlHaj Mahmoud SA. The Fok1 vitamin D receptor gene polymorphism and 25(OH) D serum levels and prostate cancer among Jordanian men. Asian Pac J Cancer Prev. 2015;16:2227–30. doi: 10.7314/apjcp.2015.16.6.2227. [DOI] [PubMed] [Google Scholar]
- 17.Yousaf N, Afzal S, Hayat T, et al. Association of vitamin D receptor gene polymorphisms with prostate cancer risk in the Pakistani population. Asian Pac J Cancer Prev. 2014;15:10009–13. doi: 10.7314/apjcp.2014.15.22.10009. [DOI] [PubMed] [Google Scholar]
- 18.Rowland GW, Schwartz GG, John EM, Ingles SA. Protective effects of low calcium intake and low calcium absorption vitamin D receptor genotype in the California Collaborative Prostate Cancer Study. Cancer Epidemiol Biomarkers Prev. 2013;22:16–24. doi: 10.1158/1055-9965.EPI-12-0922-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bai Y, Yu Y, Yu B, et al. Association of vitamin D receptor polymorphisms with the risk of prostate cancer in the Han population of Southern China. BMC Med Genet. 2009;10:125. doi: 10.1186/1471-2350-10-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holt SK, Kwon EM, Peters U, Ostrander EA, Stanford JL. Vitamin D pathway gene variants and prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2009;18:1929–33. doi: 10.1158/1055-9965.EPI-09-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torkko KC, van Bokhoven A, Mai P, et al. VDR and SRD5A2 polymorphisms combine to increase risk for prostate cancer in both non-Hispanic White and Hispanic White men. Clin Cancer Res. 2008;14:3223–9. doi: 10.1158/1078-0432.CCR-07-4894. [DOI] [PubMed] [Google Scholar]
- 22.Holick CN, Stanford JL, Kwon EM, Ostrander EA, Nejentsev S, Peters U. Comprehensive association analysis of the vitamin D pathway genes, VDR, CYP27B1, and CYP24A1, in prostate cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:1990–9. doi: 10.1158/1055-9965.EPI-07-0487. [DOI] [PubMed] [Google Scholar]
- 23.Mikhak B, Hunter DJ, Spiegelman D, Platz EA, Hollis BW, Giovannucci E. Vitamin D receptor (VDR) gene polymorphisms and haplotypes, interactions with plasma 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D, and prostate cancer risk. Prostate. 2007;67:911–23. doi: 10.1002/pros.20570. [DOI] [PubMed] [Google Scholar]
- 24.Li H, Stampfer MJ, Hollis JB, et al. A prospective study of plasma vitamin D metabolites, vitamin D receptor polymorphisms, and prostate cancer. PLoS Med. 2007;4:e103. doi: 10.1371/journal.pmed.0040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cicek MS, Liu X, Schumacher FR, Casey G, Witte JS. Vitamin D receptor genotypes/haplotypes and prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2006;15:2549–552. doi: 10.1158/1055-9965.EPI-06-0409. [DOI] [PubMed] [Google Scholar]
- 26.Rukin NJ, Luscombe C, Moon S, et al. Prostate cancer susceptibility is mediated by interactions between exposure to ultraviolet radiation and polymorphisms in the 5’ haplotype block of the vitamin D receptor gene. Cancer Lett. 2007;247:328–35. doi: 10.1016/j.canlet.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 27.John EM, Schwartz GG, Koo J, Van Den Berg D, Ingles SA. Sun exposure, vitamin D receptor gene polymorphisms, and risk of advanced prostate cancer. Cancer Res. 2005;65:5470–9. doi: 10.1158/0008-5472.CAN-04-3134. [DOI] [PubMed] [Google Scholar]
- 28.Mishra DK, Bid HK, Srivastava DS, Mandhani A, Mittal RD. Association of vitamin D receptor gene polymorphism and risk of prostate cancer in India. Urol Int. 2005;74:315–8. doi: 10.1159/000084429. [DOI] [PubMed] [Google Scholar]
- 29.Hayes VM, Severi G, Padilla EJ, et al. Genetic variants in the vitamin D receptor gene and prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2005;14:997–9. doi: 10.1158/1055-9965.EPI-04-0660. [DOI] [PubMed] [Google Scholar]
- 30.Oakley-Girvan I, Feldman D, Eccleshall TR, et al. Risk of early-onset prostate cancer in relation to germ line polymorphisms of the vitamin D receptor. Cancer Epidemiol Biomarkers Prev. 2004;13:1325–30. [PubMed] [Google Scholar]
- 31.Tayeb MT, Clark C, Haites NE, Sharp L, Murray GI, McLeod HL. Vitamin D receptor, HER-2 polymorphisms and risk of prostate cancer in men with benign prostate hyperplasia. Saudi Med J. 2004;25:447–51. [PubMed] [Google Scholar]
- 32.Cheteri MB, Stanford JL, Friedrichsen DM, et al. Vitamin D receptor gene polymorphisms and prostate cancer risk. Prostate. 2004;59:409–18. doi: 10.1002/pros.20001. [DOI] [PubMed] [Google Scholar]
- 33.Bodiwala D, Luscombe CJ, French ME, et al. Polymorphisms in the vitamin D receptor gene, ultraviolet radiation, and susceptibility to prostate cancer. Environ Mol Mutagen. 2004;43:121–7. doi: 10.1002/em.20000. [DOI] [PubMed] [Google Scholar]
- 34.Huang SP, Huang CY, Wu WJ, et al. Association of vitamin D receptor FokI polymorphism with prostate cancer risk, clinicopathological features and recurrence of prostate specific antigen after radical prostatectomy. Int J Cancer. 2006;119:1902–7. doi: 10.1002/ijc.22053. [DOI] [PubMed] [Google Scholar]
- 35.Chokkalingam AP, McGlynn KA, Gao YT, et al. Vitamin D receptor gene polymorphisms, insulin-like growth factors, and prostate cancer risk: a population-based case-control study in China. Cancer Res. 2001;61:4333–6. [PubMed] [Google Scholar]
- 36.Correa-Cerro L, Berthon P, Hussler J, et al. Vitamin D receptor polymorphisms as markers in prostate cancer. Hum Genet. 1999;105:281–7. doi: 10.1007/s004390051102. [DOI] [PubMed] [Google Scholar]
- 37.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 38.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48. [PubMed] [Google Scholar]
- 39.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen L, Davey Smith G, Evans DM, et al. Genetic variants in the vitamin D receptor are associated with advanced prostate cancer at diagnosis: findings from the prostate testing for cancer and treatment study and a systematic review. Cancer Epidemiol Biomarkers Prev. 2009;18:2874–81. doi: 10.1158/1055-9965.EPI-09-0544. [DOI] [PubMed] [Google Scholar]
- 41.Gilbert R, Bonilla C, Metcalfe C, et al. Associations of vitamin D pathway genes with circulating 25-hydroxyvitamin-D, 1,25-dihydroxyvitamin-D, and prostate cancer: a nested case-control study. Cancer Causes Control. 2015;26:205–18. doi: 10.1007/s10552-014-0500-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krishnan AV, Peehl DM, Feldman D. The role of vitamin D in prostate cancer. Recent Results Cancer Res. 2003;164:205–21. doi: 10.1007/978-3-642-55580-0_15. [DOI] [PubMed] [Google Scholar]
- 43.Lou YR, Qiao S, Talonpoika R, Syvala H, Tuohimaa P. The role of vitamin D3 metabolism in prostate cancer. J Steroid Biochem Mol Biol. 2004;92:317–25. doi: 10.1016/j.jsbmb.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 44.Gandini S, Gnagnarella P, Serrano D, Pasquali E, Raimondi S. Vitamin D receptor polymorphisms and cancer. Adv Exp Med Biol. 2014;810:69–105. doi: 10.1007/978-1-4939-0437-2_5. [DOI] [PubMed] [Google Scholar]
- 45.Kstner K, Denzer N, Müller CS, Klein R, Tilgen W, Reichrath J. The relevance of vitamin D receptor (VDR) gene polymorphisms for cancer: a review of the literature. Anticancer Res. 2009;29:3511–36. [PubMed] [Google Scholar]
- 46.Eisenhardt A, Scherag A, Jckel KH, Reis H, Rübben H, Siffert W. Lack of association of the genotype in the GNAS Fok I polymorphism and prostate cancer. Urol Int. 2011;87:80–6. doi: 10.1159/000325398. [DOI] [PubMed] [Google Scholar]
- 47.Xiao M, Guo L, Wang T, et al. Interleukin-1B-31T/C promoter polymorphism and chronic obstructive pulmonary disease risk: a meta-analysis. Arch Med Sci. 2014;10:434–8. doi: 10.5114/aoms.2014.43737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zuo L, Zhang LF, Wu XP, et al. Association of a common genetic variant in prostate stem cell antigen with cancer risk. Arch Med Sci. 2014;10:425–33. doi: 10.5114/aoms.2014.43736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ioannidis JP. Effect of the statistical significance of results on the time to completion and publication of randomized efficacy trials. JAMA. 1998;279:281–6. doi: 10.1001/jama.279.4.281. [DOI] [PubMed] [Google Scholar]
- 50.Schatzl G, Gsur A, Bernhofer G, et al. Association of vitamin D receptor and 17 hydroxylase gene polymorphisms with benign prostatic hyperplasia and benign prostatic enlargement. Urology. 2001;57:567–72. doi: 10.1016/s0090-4295(00)01004-9. [DOI] [PubMed] [Google Scholar]
- 51.Bousema JT, Bussemakers MJ, van Houwelingen KP, et al. Polymorphisms in the vitamin D receptor gene and the androgen receptor gene and the risk of benign prostatic hyperplasia. Eur Urol. 2000;37:234–8. doi: 10.1159/000020124. [DOI] [PubMed] [Google Scholar]