Figure 1.
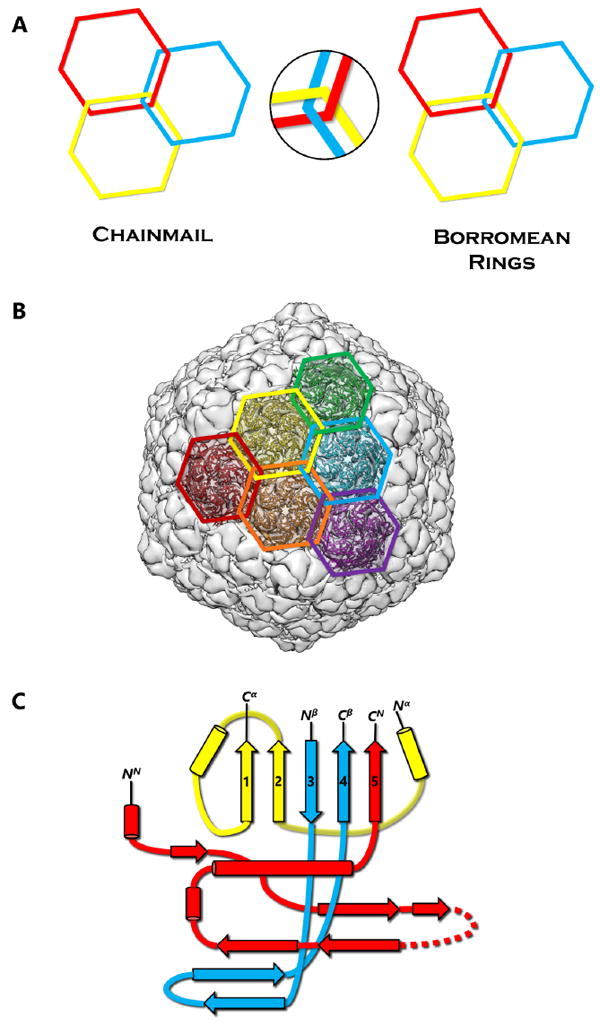
Protein Chainmail and the HK97-like fold. (A) Concept of chainmail versus Borromean rings. Breaking one ring in a chainmail would not affect the integrity of the whole, unlike the case of Borromean rings. (B) Protein chainmail of HK97. Chainmail is a structural organization of concatenated rings found in the capsids of icosahedral dsDNA viruses, and allow capsids to withstand internal forces exerted by the dsDNA. The interlocking rings of protein chainmail resemble armor constructed of metal rings worn by medieval knights during times of battle. (C) Basic building blocks of the HK97-like fold include the N (red), α (yellow), and β (blue) primary elements. Variations of the HK97-like fold can occur when the basic building blocks are connected in different order. Additionally, extra domains can be inserted at the E-loop or at the ends of the building blocks.
