Figure 7.
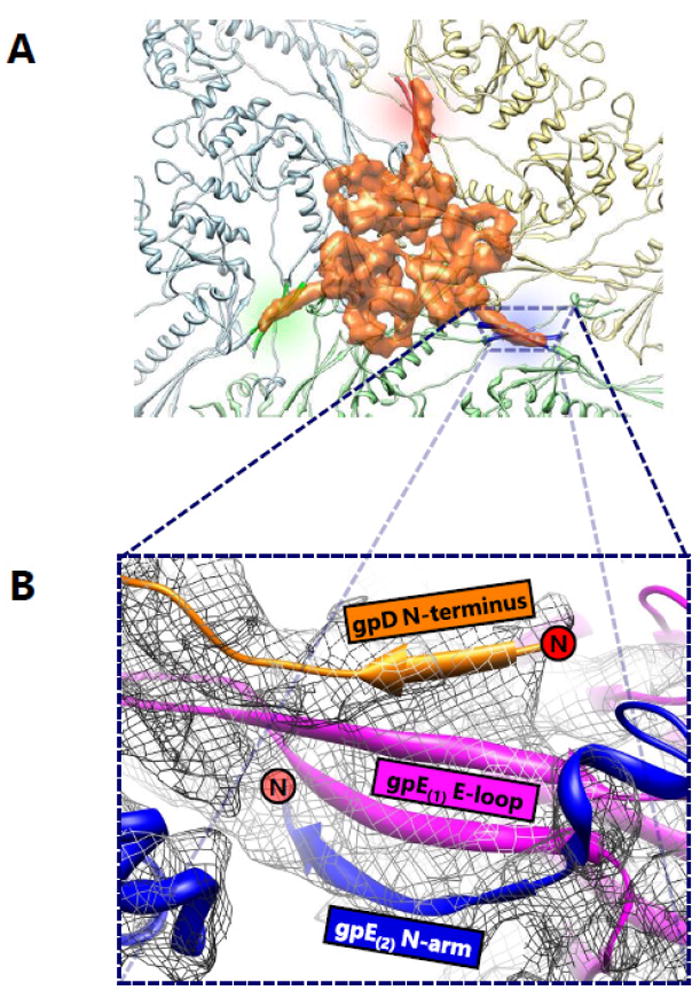
Auxiliary Protein Trimer Interactions in Phage Lambda. (A) A view of the three-fold interface of phage λ from the top. The gpD trimer (orange) is situated atop the site where subunits from three hexamers composed of gpE capsid proteins meet. The gpD trimer interacts with two subunits from each hexamer. In this sense, the gpD trimer acts as a molecular staple and anchors six gpE subunits together. (B) A zoomed-in view of the stabilizing interaction of a single gpD monomer. A four-stranded β sheet is formed from a strand from the gpD N-terminus (orange), two strands from the E-loop of a gpE subunit (magenta), and another strand from the N-arm of an adjacent gpE subunit (blue). The N-termini of gpD and the N-arm of gpE are denoted by red circles. Adapted from [8] with permission from the publisher.
