Figure 1. Protein S-Nitrosylation in NO Signaling under Neurodegenerative Conditions.
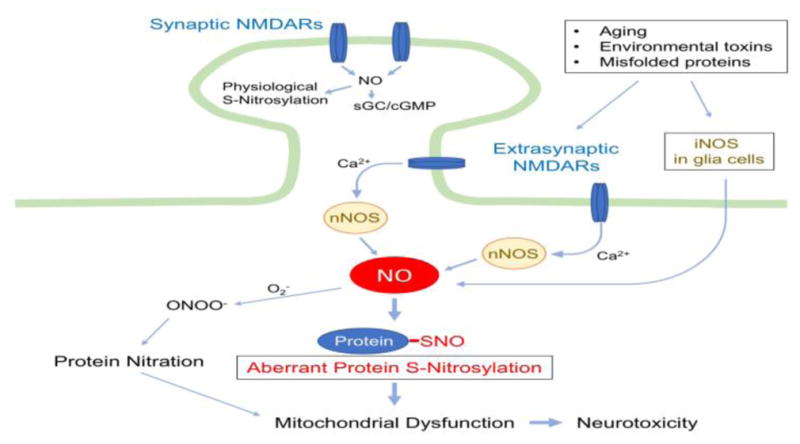
Upon activation by its dual agonists, glutamate and glycine (or D-serine), NMDA receptors (NMDARs) allow entry of Ca2+ into the cytosol of the postsynaptic cell. While synaptic NMDARs mediate synaptic plasticity and neuroprotection, hyperactivation of extrasynaptic NMDARs (eNMDARs) enhances neurotoxicity [94]. Under neurodegenerative conditions, increased Ca2+ influx due predominantly to eNMDAR overactivation stimulates excessive NO production from nNOS. Additionally, NO derived from iNOS in astrocytes augments nitrosative stress. Subsequently, the covalent addition of NO-related species to a reactive cysteine sulfhydryl (or thiolate) group in a target protein leads to the formation of S-nitrosylated (SNO) proteins. For example, pathologically high levels of NO can trigger aberrant S-nitrosylation of mitochondrial proteins, resulting in dysfunction in mitochondrial metabolism and dynamics. NO can also react with superoxide (O2−) to yield peroxynitrite (ONOO−), leading to tyrosine nitration. In contrast, under physiological conditions, synaptic NMDAR activity maintains relatively low levels of NO production, activating soluble guanylyl cyclase (sGC)/cGMP pathways and neuroprotective protein S-nitrosylation-mediated pathways.
