Abstract
Heat Shock Protein 70 (Hsp70) is a molecular chaperone required for protein folding, cell viability and cancer cell proliferation. Recent studies suggest that Hsp70 phosphorylation may regulate important cellular processes such as cell cycle progression, apoptosis, protein degradation and resistance to anticancer therapeutics.
Keywords: Chaperones, Phosphorylation, Hsp70, kinases, cancer
Classic modes of Hsp70 regulation
Heat Shock Protein 70 (Hsp70) is a well-conserved molecular chaperone involved in the folding of proteins, modulation of protein-protein interactions and degradation of damaged proteins (for in depth discussion of Hsp70 function, please see (1)). Previous studies have focused on changes in expression under stress conditions and regulation by selective binding of helper molecules known as co-chaperones. There are two major Hsp70 isoforms in mammalian cells, Hsc70 and Hsp70. While Hsc70 is constitutively expressed and provides essential housekeeping roles, Hsp70 levels are stressed induced, rising to bind and refold denatured proteins. This process is tightly regulated by a master transcription factor, HSF1 (for more details see (2)).
While this is still considered the main way of controlling Hsp70 activity in cells, mounting evidence points another strategy, Hsp70 phosphorylation. Patterns of specific Hsp70 phosphorylation, known as “the chaperone code” may fine-tune Hsp70 function (Fig.1 and (3)). Below we discuss recent updates in the efforts to understand the role of Hsp70 phosphorylation in its in vivo function.
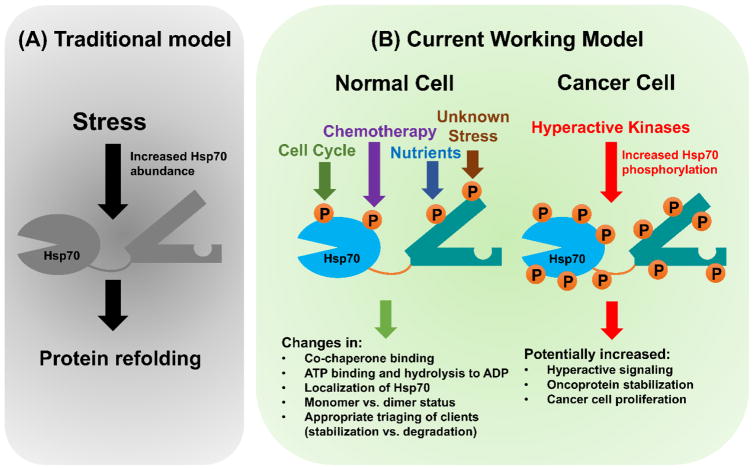
Figure 1. Models of Hsp70 regulation.
(A) Hsp70 regulation through altered protein expression. Cell stress (such as heat shock) triggers protein unfolding and stimulates increased Hsp70 expression. Hsp70 binds and refolds proteins restoring homeostasis. (B) Hsp70 regulation through phosphorylation. In normal cells, a variety of stimuli such as cell cycle progression, exposure to chemotherapeutic drugs, nutrient availability and others yet to be determined alter Hsp70 phosphorylation. These alter the ability of Hsp70 to ultimately fold and degrade client proteins. In cancer cells, kinase activity becomes misregulated as a result of random mutation. Our current theory is that this increased Hsp70 phosphorylation may disrupt the balance of the chaperone code, leading to increased cancer protein stability and thus cancer proliferation.
Identification of Hsp70 phosphorylation sites
In recent years, identification of phosphorylation sites has been greatly accelerated through the use of Mass spectrometry. Global and targeted phosphoproteomics have uncovered 53 phosphorylation sites on Hsp70 (see PhosphoSitePlus (http://www.Phosphosite.org) and Fig.2). While these studies pinpoint sites of phosphorylation, they speak nothing towards their regulation of role in chaperone function. Bioinformatics techniques for prioritizing these in terms of functional importance have met with success. Beltrao et al. compiled nearly 200,000 phosphorylation sites for a variety of proteins that included Hsp70, predicting those most likely to mediate protein-protein interactions and enzyme activity. 313 phosphosites were identified on Hsp70 isoforms across 11 species and their enrichment analysis highlighted two significant hot spots: one adjacent to the nucleotide binding domain (Region 1) and the other near the substrate binding domain (Region 2). In these two hotspots, five phosphorylation sites (T36, T38 for Region 1 and T492, S495 and T499 for Region 2) were examined as putative sites of chaperone regulation. Each of these sites when mutated to alanine or aspartic acid impacted cellular thermotolerance and caused defective Ssa1-polysome binding when expressed in yeast (4).
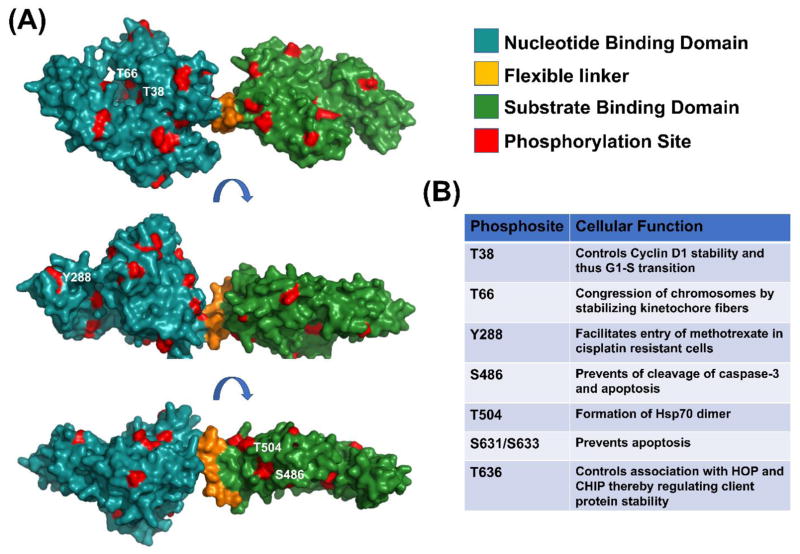
Figure 2. Currently known phosphorylation sites on Hsp70.
(A) Observed Hsp70 phosphorylation sites (from PhosphoSitePlus database) have been mapped onto the predicted structure of Hsc70 based on PDB structure 2KHO (marked in red). Three different rotated viewpoints are shown for clarity. (B) Table summarizing Hsp70 phosphorylation sites that have a described role. Further information on these sites can be found in references (5–11).
Regulation of cell cycle progression through Hsp70 phosphorylation
Among the many known functions of Hsp70, a complex role in cell cycle progression has been described. In budding yeast, Hsp70 binds and regulates the stability of the G1 cyclin Cln3 (Cyclin D1). Cln3 encodes a J-like domain that competes with the co-chaperone Ydj1 for binding. Ydj1 displaces the Cln3 bound to Hsp70 during the G1-S transition, allowing Cln3 transit to the nucleus, driving cell cycle progression. This molecular switch is directly controlled by Hsp70 T36 phophorylation. During times of nutrient scarcity, the Pho85 Cyclin-Dependent Kinase (CDK) phosphorylates T36 causing Hsp70-Ydj1 dissociation and Cln3 degradation, stalling cells in G1 until fresh nutrients become available. In a conserved mechanism, phosphorylation of mammalian Hsp70 on the equivalent residue (T38) leads to chaperone-mediated destruction of Cyclin D1 and repression of cell cycle-controlled signaling (5).
Polo-like kinase 1 (Plk1), an important mitotic kinase, can also phosphorylate Hsp70. Treatment of HeLa S3 cells with arsenic trioxide (ATO) produced rapid Plk1-mediated Hsp70 phosphorylation at five residues: T13, S362, T226, S631 and S633. Cells expressing Hsp70S631A/S633A were significantly more prone to ATO-induced mitotic arrest than wild-type cells, suggesting Plk1-mediated phosphorylation of Hsp70 serves a protective role against cell death by apoptosis. Hsp70 phosphorylated at Serine 631 and Serine 633 co-localized with Plk1 at the centrosome, suggesting a role for Hsp70 phosphorylation in mitotic arrest. This association led to an increase in microtubule stability and elongation of mitotic spindles. Hence, the phosphorylation of Hsp70 at S631 and S633 activates Hsp70 as a centrosomal chaperone (6). Although the mechanism underpinning this cellular survival strategy remains unclear, the inhibition of Hsp70 phosphorylation or Plk1 inhibition might serve as a key to increase efficiency of ATO as a chemotherapeutic drug.
Plk1 is not the only kinase to phosphorylate Hsp70 to ensure correct spindle function. Nek6 phosphorylates Hsp70 at T66 leading to localization of Hsp72 at the mitotic spindles and aids in the congression of chromosomes by stabilizing the kinetochore fibers via recruitment of the ch-TOG and TACC3 proteins. Hsp70T66A fails to localize at spindle poles resulting in kinetochore fiber destabilization and abnormal mitotic progression (7).
Hsp 70 phosphorylation alters cellular resistance to chemotherapeutic drugs
Given that Hsp90 phosphorylation can mediate cancer cell growth and cellular resistance to cancer chemotherapies, it has been proposed that Hsp70 phosphorylation may have similar effects. Phosphorylation of Hsp70 on Y288 regulates the binding affinity of Hsc70 to methotrexate and reduced folate carrier protein (RFC), which is a primary transporter of folate and anti-folate drugs leading to the entry of methotrexate into the cell (8). Further studies are required to identify the key players that mediate the phosphorylation and de-phosphorylation events. Going forward, it may be possible to alter Y288 phosphorylation status clinically to reduce drug resistance in leukemic cells.
Control of Apoptosis via phosphorylation of Hsp70
Retinoic acid induced 16 protein (RAI16) is activated upon serum stimulation and forms a scaffold, binding both protein kinase A (PKA) and Hsp70. Bringing PKA and Hsp70 into proximity promotes PKA-mediated phosphorylation of Hsp70 on S486, preventing cleavage of caspase-3 and apoptosis. In contrast, upon exposure to elevated cAMP levels RAI16 becomes phosphorylated at S325 by PKA and the recruitment of adaptor protein 14-3-3Θ occurs at this site. This recruitment inhibits Hsp70 phosphorylation at S486 and promotes apoptosis (9).
Regulation of Hsp70 dimerization by phosphorylation
In contrast to Hsp90, Hsp70 is primarily monomeric. An elegant study combining crosslinking-mass spectrometry and structure modeling determined that phosphorylation of T504 on mammalian Hsp70 is critical for Hsp70 dimerization and promotes a client-loading complex consisting of Hsp90, Hsp70 and Hsp40. This complex is remarkably stable and may act as a “shelter” for clients while they are folding (10). It remains to be seen whether this phosphorylation is constitutive or varies depending on cellular conditions of client protein binding.
Regulation of Hsp70 client triaging by phosphorylation
Client proteins typically go through several rounds of chaperone-mediated folding during their lifespan. If Hsp70 binds a non-refoldable protein, it will target this client for degradation via the proteasome. While CHIP (a C-terminal associated co-chaperone) is important for this process, the process that dictates whether Hsp70 should fold or assist in degradation remains unclear.
Phosphorylation of the C-terminal substrate-binding domains of Hsp70 and Hsp90 regulates the client triaging process. C-terminal phosphorylation of Hsp70 and Hsp90 (T636 in Hsp70) leads to increased association with the HOP co-chaperone and increased client protein stability. Loss of this phosphorylation promotes loss of HOP binding and increased CHIP binding, resulting in client degradation. Given that T636 phosphorylation seems especially prevalent in proliferating cancer cells, this could be a prime determinant of oncoprotein stability, driving cancer cell growth (11). More recent studies have shown that T636 phosphorylation, while greatly impacting HOP and CHIP interactions had little effect on the binding of other co-chaperones such as DnaJC7, FKBP51 and FKBP52, even though they bind to Hsp70 at roughly the same location (12). The regulation of the selectivity of binding to co-chaperones remains obscure, but an interesting facet of chaperone quality control.
Concluding Remarks
Many of the current studies lack mechanistic insight into the kinases and the upstream signaling processes that may be required for the specific phosphorylation of Hsp70 to occur in the first place. Even when the upstream activation processes and downstream effects of each site are determined, crosstalk between these sites must be considered.
Studies of kinase-chaperone interactions are confounded by reciprocal regulation; kinases can phosphorylate the very chaperones that stabilize them. Teasing apart these interactions will be difficult but ultimately rewarding. While Hsp70 is highly abundant, kinases are expressed at much lower levels to ensure signaling fidelity. This raises an important question of stoichiometry as to what proportion of Hsp70 is phosphorylated at any given time. It is likely that there are specific pools of Hsp70 in different cellular compartments/regions containing high local concentrations of specific phosphorylation sites but at low global concentration. It is clear that a complete understanding of the chaperone code will take time given its multilayered complexity.
Acknowledgments
This project was supported by NCI R15CA208773.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kampinga HH, Craig EA. The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat Rev Mol Cell Biol. 2010;11:579–592. doi: 10.1038/nrm2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akerfelt M, Morimoto RI, Sistonen L. Heat shock factors: integrators of cell stress, development and lifespan. Nat Rev Mol Cell Biol. 2010;11:545–555. doi: 10.1038/nrm2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cloutier P, Coulombe B. Regulation of molecular chaperones through post-translational modifications: decrypting the chaperone code. Biochim Biophys Acta. 2013;1829:443–454. doi: 10.1016/j.bbagrm.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beltrao P, et al. Systematic functional prioritization of protein posttranslational modifications. Cell. 2012;150:413–425. doi: 10.1016/j.cell.2012.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Truman AW, et al. CDK-dependent Hsp70 Phosphorylation controls G1 cyclin abundance and cell-cycle progression. Cell. 2012;151:1308–1318. doi: 10.1016/j.cell.2012.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen YJ, et al. HSP70 colocalizes with PLK1 at the centrosome and disturbs spindle dynamics in cells arrested in mitosis by arsenic trioxide. Arch Toxicol. 2014;88:1711–1723. doi: 10.1007/s00204-014-1222-x. [DOI] [PubMed] [Google Scholar]
- 7.O’Regan L, et al. Hsp72 is targeted to the mitotic spindle by Nek6 to promote K-fiber assembly and mitotic progression. J Cell Biol. 2015;209:349–358. doi: 10.1083/jcb.201409151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu T, et al. Identification and characterization of a 66-68-kDa protein as a methotrexate-binding protein in murine leukemia L1210 cells. Cell Stress Chaperones. 2013;18:223–234. doi: 10.1007/s12192-012-0376-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding CL, et al. Anchoring of both PKA-RIIalpha and 14-3-3theta regulates retinoic acid induced 16 mediated phosphorylation of heat shock protein 70. Oncotarget. 2015;6:15540–15550. doi: 10.18632/oncotarget.3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morgner N, et al. Hsp70 forms antiparallel dimers stabilized by post-translational modifications to position clients for transfer to Hsp90. Cell Rep. 2015;11:759–769. doi: 10.1016/j.celrep.2015.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muller P, et al. C-terminal phosphorylation of Hsp70 and Hsp90 regulates alternate binding to co-chaperones CHIP and HOP to determine cellular protein folding/degradation balances. Oncogene. 2013;32:3101–3110. doi: 10.1038/onc.2012.314. [DOI] [PubMed] [Google Scholar]
- 12.Assimon VA, Southworth DR, Gestwicki JE. Specific Binding of Tetratricopeptide Repeat Proteins to Heat Shock Protein 70 (Hsp70) and Heat Shock Protein 90 (Hsp90) Is Regulated by Affinity and Phosphorylation. Biochemistry. 2015;54:7120–7131. doi: 10.1021/acs.biochem.5b00801. [DOI] [PMC free article] [PubMed] [Google Scholar]