FIGURE 1.
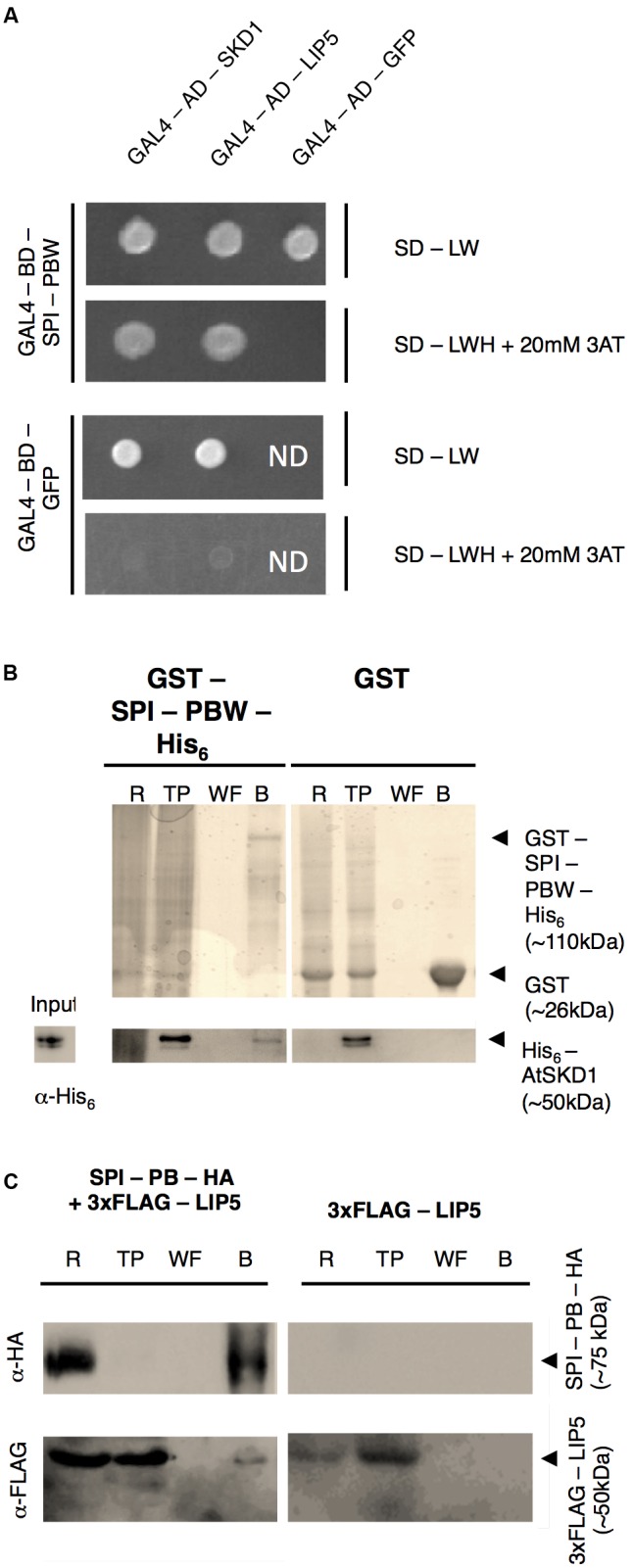
SPI interacts with SKD1 and LIP5. (A) Yeast two-hybrid interactions between SPI-PBW, SKD1, and LIP5. Top: double transformed yeast cells on dropout medium lacking leucine (-L) and tryptophan (-W). Bottom: interaction between GAL4-Binding Domain (BD) fusions of SPI-PBW, and SKD1 or LIP5, N-terminally fused to the GAL4 Activation Domain (AD), on dropout medium lacking leucine (-L), tryptophan (-W) and histidine (-H), supplemented with 20 mM 3-Aminotrizole (3AT). GAL4-AD-GFP (Green Fluorescent Protein) and GAL4-BD-GFP vectors served as negative controls. ND, not determined. (B) Co-precipitation of bacterially expressed SPI-PBW and SKD1. Top: Purifications of GST-SPI-PBW-His6 (∼110 kDa) and the negative control GST (∼26 kDa) are shown on Coomassie stained gels. Bottom: Input of purified His6-SKD1 (∼50 kDa) and co-precipitations of His6-SKD1/GST-SPI-PBW-His6 detected by α-His6 antibody staining. No co-precipitation was observed between GST and His6-SKD1. Expected protein sizes are indicated by arrowheads. R, raw extract; TP, throughput; WF, last wash fraction; B, beads fraction. (C) Co-immunoprecipitation of SPI-PB-HA and 3xFLAG-LIP5 from lysates of transfected N. benthamiana leaves. Top: Immunoprecipitation of SPI-PB-HA (∼75 kDa) was detected by α-HA antibody staining on a Western blot. Bottom: 3xFLAG-tagged LIP5 (∼50 kDa) was detected in R, TP and the B of SPI-PB-HA co-transfected leaves by α-FLAG antibody staining on Western blots. 3xFLAG-tagged LIP5 alone was not precipitated with α-HA-beads. Expected protein sizes are indicated by arrowheads. ProteinA beads were included as a negative control for all proteins tested (Supplementary Figure S1B).
