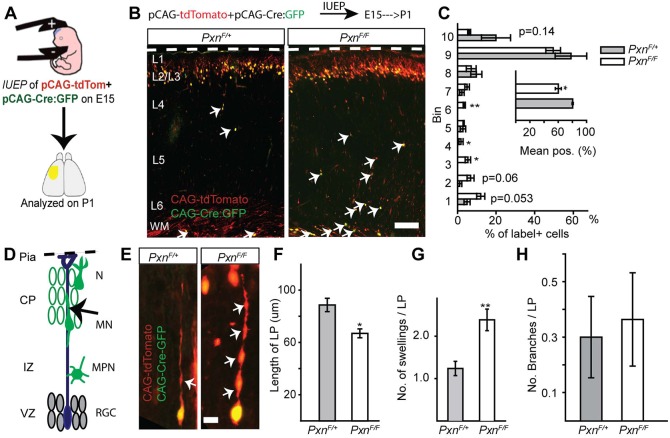
Fig. 6.
Cell-autonomous deletion of paxillin alters neuronal positioning and morphology. (A) In utero electroporation of pCAG-Cre:GFP+pCAG-tdTomato into PxnF/+ (control) or PxnF/F (paxillin-deficient) was performed at E15. (B) Representative image of control and paxillin-deficient neurons analyzed at P1. (C) Analysis of cell position across the cortical wall by bins (bin 1 includes WM and bin 10 includes L1). The mean cell position of the paxillin-deficient group is 20% deeper than control. (D) Schematic of radial migration and a bipolar migrating neuron (arrow) analyzed in the cortical plate (CP). (E) A control (PxnF/+) migrating neuron with an extended leading process (left panel). Cre-mediated deletion of paxillin shortened and increased the number of swellings (arrows) in the leading process (right panel). (F) Quantification of leading process lengths (n=15 cells per group). (G) Quantification of leading process swellings (n=21 cells for PxnF/+, n=26 cells for PxnF/F). (H) The number of leading process branches was indistinguishable between the groups (n=20 cells for PxnF/+, n=22 cells for PxnF/F). CP, cortical plate; IZ, intermediate zone; WM, white matter; VZ, ventricular zone; RGC, radial glial cells; MPN, multipolar neurons; MN, migrating neurons; N, differentiated neurons. *P<0.05, **P<0.01. Scale bars: 100 µm in B; 10 µm in E.