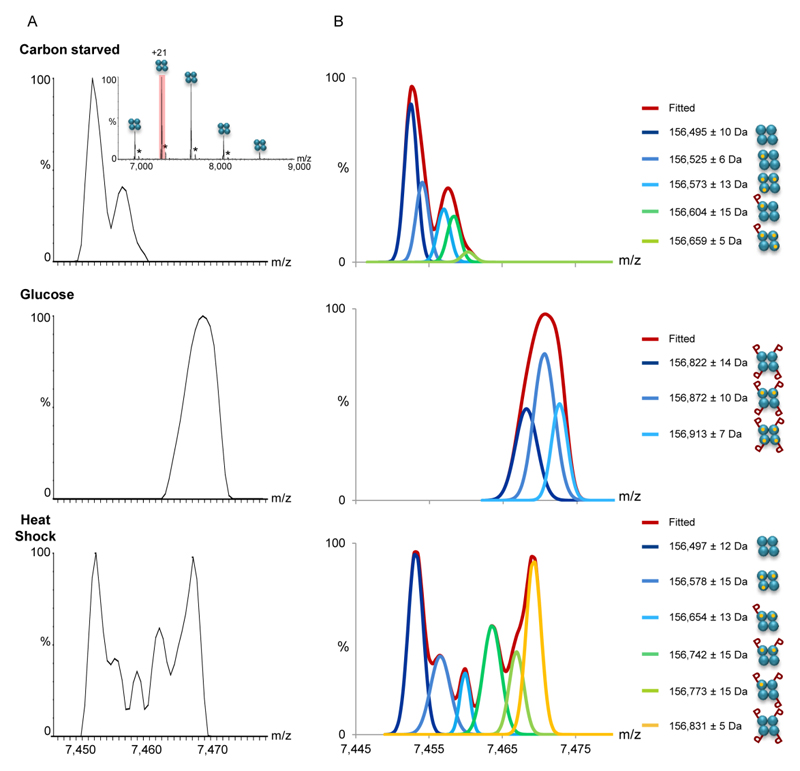
Figure 2. MS1 analysis indicates that changes in growth conditions are accompanied by differential PTMs.
(A) The FBP1 complex was FLAG-affinity purified from yeast grown in the absence of glucose (top panel), after a shift to glucose (middle panel), or after exposure to heat shock (bottom panel). Mass spectra of purified FBP1 complexes were acquired on the modified Orbitrap™ system, at conditions favoring the preservation of non-covalent interactions, thus preserving the homotetrameric structure (inset). Enlarged view of the +21 charge state reveals differential modifications in response to the different treatments. Black asterisks correspond to homotetrameric FBP1 complexes non-covalently associated with a FLAG peptide adduct. (B) Deconvolution of the measured spectra aided the assignment of the peaks. Assignment was performed by analysis of the deconvoluted spectra and mass measurements. The identified FBP1 species are shown on the right. The results show that the addition of glucose shifted the complex to a uniformly phosphorylated state, at four positions. In contrast, the coexistence of species corresponding in mass to 0 to 4 phosphorylation events was detected, following yeast exposure to heat shock. As expected, association with Mg2+ ions was also obtained. Each FBP1 subunit is graphically depicted as cyan circle. Mg2+ ions are indicated as small orange circles, and phosphorylation is labeled as “P”.