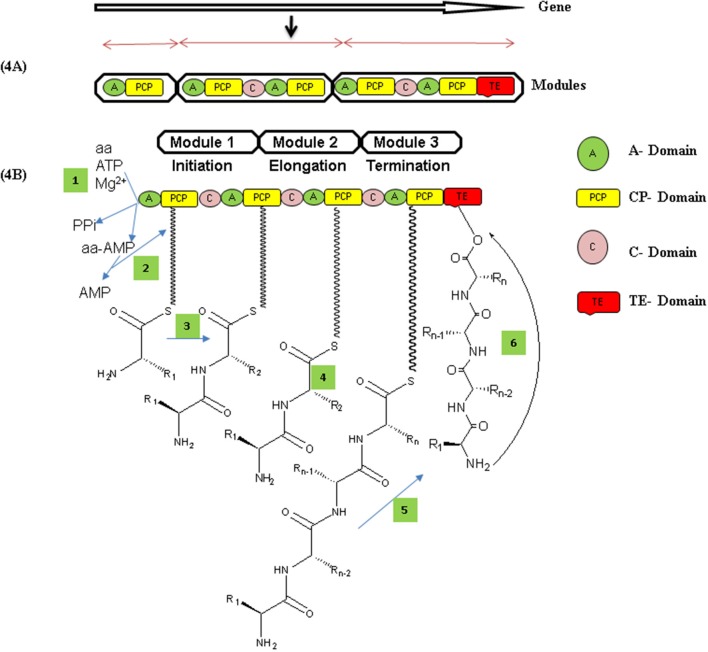
Figure 4.
(A) Organization of modules and their domains in nonribosomal peptide synthetase enzyme. Each module contains their catalytic domains that catalyze activities, substrate activation (A-domain), covalent loading (CP-domain), and peptide bond formation (C-domain). The first modules always lacks a C domain and is used to initiate nonribosomal peptide synthesis, while those harboring a C-domain qualify for elongation and modules with thioesterase domains (TE) usually in the last domain, for termination of peptide product from enzyme through cyclization or hydrolysis (Prieto et al., 2012). (B) Mechanism of nonribosomal peptide (NRP) synthesis Adenylation domain (A) activates amino acid as aminoacyl-AMP and transfer to PCP domain which condenses coming amino acids by forming peptide bonds. Structural modifications mostly operate by epimerization domains which converts L-amino acid to D-amino acid and vice a versa. Peptide chain thus transfers to TE domain by transesterification reaction by PCP. Finally, TE domain catalyzed product release (NRPs) by either hydrolysis or macrocyclization (Condurso and Bruner, 2012).