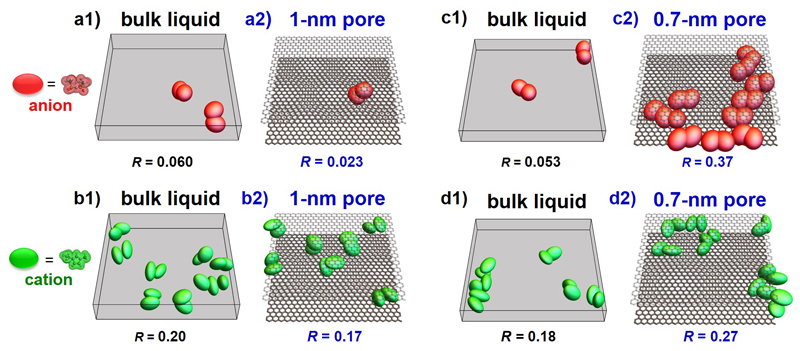
Figure 3. Enhanced co-ion pair formation in carbon nanopores of decreasing pore size.
(a1, b1, c1, d1), Snapshots of co-ion pairs of anions (a1, c1) and cations (b1, d1) for EMI-TFSI bulk liquid (opposite charge ion pairs are not shown since they are the regular pattern of such ionic liquids). The co-ion pairs are extracted from bulk EMI-TFSI snapshots in 1-nm (a1, b1) and 0.7-nm slit spaces (c1, d1). (a2, b2, c2, d2), Snapshots of anion pairs (a2, c2) and cation pairs (b2, d2) for EMI-TFSI in 1-nm (a2, b2) and 0.7-nm pores (c2, d2). R is the ratio of paired anion (or cation) number to total anion (or cation) number. EMI and TFSI ions are shown as green and red ellipsoids, respectively. For narrower pores, repulsion of ions with the same charge is suppressed by electronic screening of Coulomb interactions by the conducting walls, which leads to an enhancement in the number of co-ion pairs. Note that here co-ions designate ions of the same sign as the central ion and that the pore walls have no net charges since they are not polarized.