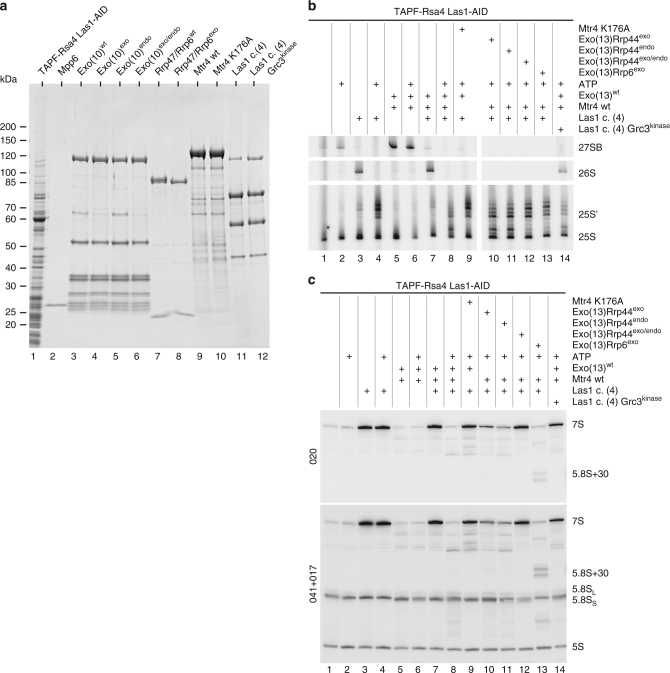
Fig. 5.
Complete cycle of in vitro ITS2 processing by the combined activities of the Las1 complex and nuclear exosome. a Analysis of the protein samples used in the complete in vitro ITS2-processing assay. Substrate 27SB pre-rRNA was introduced by addition of pre-60S particles affinity-purified via TAPF-Rsa4 from a LAS1-AID degron strain. The yeast exosome subunits and Mtr4 were expressed in E. coli and affinity-purified from bacterial cell lysates. Exo(10) comprises the exosome core plus Rrp44. The Las1 complex (Rat1, Grc3, Las1, Rai1, from top to bottom) was overexpressed in yeast and TAP-purified via the split-tag approach (Las1-TpA/Grc3-Flag), yielding a wt and a mutant Las1 complex with a defective Grc3 kinase. The indicated eluates were analysed by SDS-PAGE and Coomassie staining. b Primer extension for detection of 27SB pre-rRNA, 26S pre-rRNA, 25S’ pre-rRNA and 25S rRNA. c Northern blotting for detection of 7S pre-rRNA, 5.8S rRNA and 5S rRNA after the in vitro processing reaction. Processing of 27SB substrate pre-rRNA present in pre-60S particles (affinity-purified via TAPF-Rsa4 from LAS1-AID degron strain) using the recombinant exosome and its cofactor Mtr4, and affinity-purified Las1 complex, either wt or harbouring the Grc3 kinase mutant. The in vitro reactions contained the purified proteins and ATP as indicated. 7S pre-rRNA was detected with two different probes. 5S rRNA served as a loading control