Figure 2.
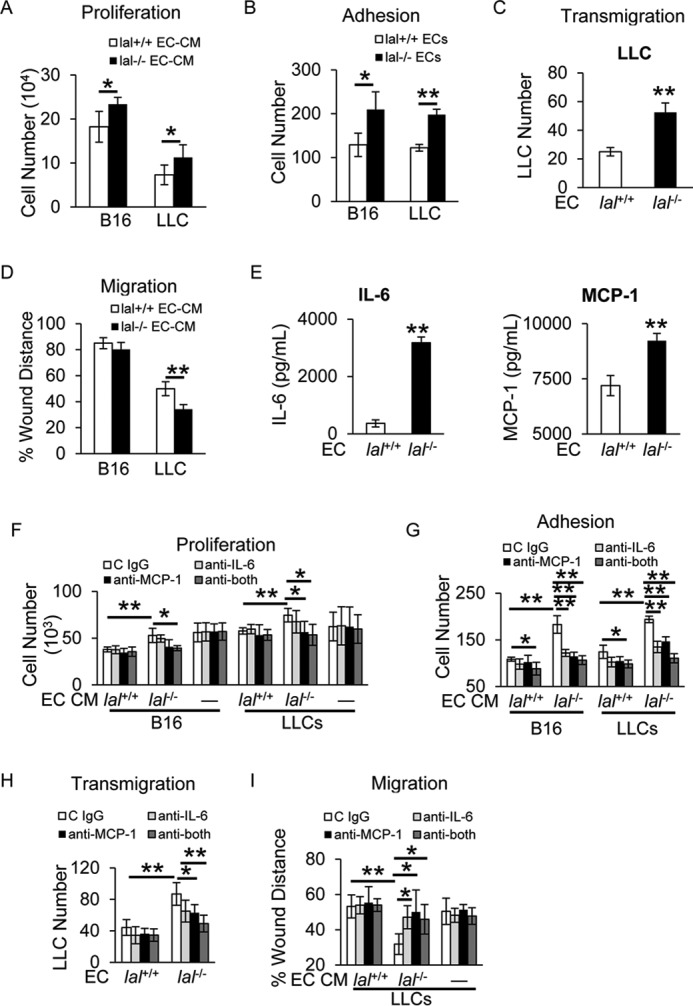
lal−/− ECs stimulated tumor cell proliferation, migration, adhesion, and transendothelial migration in vitro. A, B16 melanoma or LLC cells (2 × 104) were co-cultured with CM of lal+/+ or lal−/− ECs in vitro for 72 h for tumor cell enumeration. B, CMFDA-labeled tumor cell (B16 melanoma or LLC cells, 1 × 104) adhesion to a lal+/+ or lal−/− EC monolayer. Total cell number of five fields in each well was quantified using a fluorescence microscope. C, CMFDA-labeled LLC transendothelial migration across the lal+/+ or lal−/− EC monolayers in the Transwell assay. LLC cells migrated to the lower chamber were counted. D, tumor cell migration after lal+/+ or lal−/− EC-CM treatment by in vitro wound healing assay at 15 h in the presence of mitomycin C. E, secretion of IL-6 and MCP-1 by ECs in the culture medium was measured by ELISA. F, antibody neutralization against IL-6 and MCP-1 individually or in combination in ECs; IgG was the control. The CM was harvested and used to treat B16 melanoma or LLC cells (5 × 103) in vitro for 72 h for tumor cell enumeration. To exclude the potential effects of neutralizing antibody on tumor cells, neutralizing antibodies or control (C) IgG were added to tumor cell culture medium directly. G, CMFDA-labeled tumor cell (B16 melanoma or LLC cells, 1 × 104) adhesion to lal+/+ or lal−/− EC monolayers that were pretreated with neutralizing antibody against IL-6, MCP-1 individually or in combination or control IgG. H, CMFDA-labeled LLC transendothelial migration across the lal+/+ or lal−/− ECs monolayers that were pretreated with neutralizing antibodies or control IgG. I, tumor cell migration after neutralizing antibody–pretreated EC-CM treatment was assessed by in vitro wound healing assay in the presence of mitomycin C. For all experiments, data are expressed as mean ± S.D. n = 3∼4. *, p < 0.05; **, p < 0.01.
