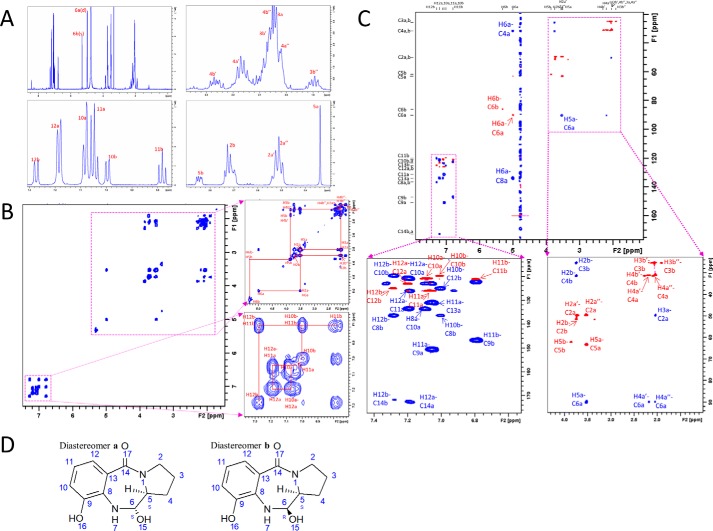
Figure 7.
Resonance assignment of kleboxymycin NMR spectra in D2O. A, 1D 1H zg30 spectrum of kleboxymycin in D2O and proton assignments. Kleboxymycin has two diastereomers a and b as carbon 6 is a chiral center after –OH addition to the double bond. B, 1H-1H COSY spectrum showing aliphatic protons' (H2–H5) cross-peaks at the region 1.8–5.4 ppm (right top panel). Aromatic protons' (H10–H12) cross-peaks were observed at the region 6.70–7.38 ppm (right bottom panel). H5 and H6 cross-peaks were also identified. C, 2D 1H–13C HMBC (blue) and HSQC (red for –CH– and pink for –CH2–) overlaid spectrum showing the assignments of carbon without protons attached (C9, C13, and C14). D, chemical structures of diastereomers a and b of kleboxymycin showing the stereo-isomerism on C6.