Abstract
Vibrio cholerae is a water-borne bacterial pathogen and causative agent of cholera. Although V. cholerae is a halophile, it can survive in fresh water, and this has a major role in cholera epidemics through consumption of contaminated water and subsequent fecal–oral spread. After dissemination from humans back into fresh water, V. cholerae encounters limited nutrient availability and an abrupt drop in conductivity but little is known about how V. cholerae adapts to, and survives in this environment. In this work, by abolishing or altering the expression of V. cholerae genes in a high-throughput manner, we observed that many osmotic shock tolerant mutants exhibited slowed or arrested growth, and/or generated a higher proportion of persister cells. In addition, we show that growth-arrested V. cholerae, including a persister subpopulation, are generated during infection of the intestinal tract and together allow for the successful dissemination to fresh water. Our results suggest that growth-arrested and persister subpopulations enable survival of V. cholerae upon shedding to the aquatic environment.
Introduction
Diarrheal disease represents the second leading cause of death in young children worldwide. Among the pathogens responsible, V. cholerae is a water-borne bacterium that causes cholera, an acute intestinal infection that produces profuse secretory diarrhea (called rice-water stool) and vomiting, which can quickly lead to severe dehydration and death if untreated. The V. cholerae life cycle includes environmental and infection stages. It resides in fresh and estuarine water in temperate zones by association with phytoplankton and the chitinous carapaces of zooplankton (Johnson, 2013) and causes epidemics through its initial consumption in contaminated water and subsequent fecal–oral spread. V. cholerae exits humans in a physiological state or set of states that prepares it for dissemination and transmission (Bourassa and Camilli, 2009). The V. cholerae genes that contribute to dissemination appear to differ from those needed for transmission (Kamp et al., 2013). After transfer from rice-water stool to fresh (pond, lake or river) water, bacteria suffer an osmotic downshift and a ~50-fold drop in conductivity, also encountering limited inorganic nutrients and carbon sources (Nelson et al., 2008). It has been proposed that V. cholerae then enters into an active but non-culturable (ANBC) state (Kell et al., 1998), in which the bacterial cells maintain their metabolic activities but lose their culturability on laboratory media (Colwell, 2000). However, the major contributors to infection from contaminated water appear to be the culturable bacteria, not the ANBC population (Nelson et al., 2008). Therefore, during cholera outbreaks, V. cholerae must adapt to the aquatic reservoir without losing its ability to infect the next host and, in the process, must also resist this harsh environment. So far, how it accomplishes this is largely unknown. Here, we used a high-throughput analysis to identify genes needed by V. cholerae to survive a hypo-osmotic shock in fresh water. We also show that V. cholerae shed from the infected host include stationary phase persister subpopulations. Our results address the importance of these subpopulations formed during infection and their impact on the dissemination of V. cholerae.
Materials and methods
Strains, media and growth conditions
E7946, a wild-type strain of V. cholerae O1 serogroup El Tor biotype (Levine et al., 1982) and its derivatives were grown in LB broth or on LB agar plates supplemented with 20 mm MgSO4 and 2 mg ml−1 sodium pyruvate; these supplements improve plating efficiency of antibiotic stressed cells (Wu et al., 2012) at 37 °C. Fresh water was collected from the Massapoag Lake in MA, USA. Its chemical composition is similar to pond water obtained from a cholera endemic area in Bangladesh (Schild et al., 2007; Nelson et al., 2008; Bourassa and Camilli, 2009; Kamp et al., 2013). Expression from the outward-facing Ptac present at one end of mTn10 used for mutagenesis was induced by the addition of isopropyl-beta-d-thiogalactopyranoside (IPTG) to a final concentration of 1 mm. Antibiotics were added when appropriate at the following concentrations: streptomycin 100 μg ml−1, gentamicin 100 μg ml−1, ampicillin 50 μg ml−1, spectinomycin 100 μg ml−1, cloramphenicol 2 μg ml−1 and kanamycin 50 μg ml−1. X-Gal was used at a concentration of 80 μg ml−1.
Fresh water challenge and persister isolation
108 colony-forming units (CFU) of E7946 or its derivatives were washed once with autoclave-sterilized fresh water and subjected to static incubation in fresh water for 15 min at 24 °C. For isolation of bacterial persisters, E7946 cultures were incubated in LB supplemented with 100 μg ml−1 gentamicin for 4 h at 37 °C with shaking. Survival was assayed by serial dilution and plating for viable CFU. Survival rate was calculated as follows: CFU/mltreated/CFU/mluntreated and converted logarithmically.
Transposon sequencing
Random insertion libraries made with mTn10 carrying either a spectinomycin, kanamycin or ampicillin resistance gene were grown until OD600 of 0.1 (exponential) or for 16 h to stationary phase (input). 108 CFU were washed and subjected to static incubation in fresh water for 15 min. The survivors were outgrown in LB broth overnight (output) and gDNA was extracted for sequencing of the mTn10 junctions using the HTML-PCR method (Lazinski and Camilli, 2013). Samples were sequenced on an Illumina HiSeq 2500 using a mTn10-specific sequencing primer. The relative abundance of each mTn10 insertion in the input and output samples was determined using the Galaxy platform available at Tufts University using custom scripts. To identify fitness changes, the Dval Genome value was used. Dval genome is calculated as: (number of reads gene X/total number of reads)/(size of gene X)/(size of the genome). Mutants were considered to be positively or negatively selected if each gene had at least three unique insertions in all input samples, and an average survival index (Dval genome output/input) ⩾4.5 or ⩽0.2 was observed, respectively. If a mutant is absent from the sample (e.g., mutants in essential genes) a Dval Genome value of #DIV/0 is shown.
Validation of genes identified as important for osmotic shock survival
Mutants were generated by splicing by overlap extension PCR (Horton et al., 1989; Dalia et al., 2014) and natural transformation. To modulate the level of expression of a gene of interest, a lacI-Ptac cassette (base pair 57 to 2517 of pDL1098 as described in (McDonough et al., 2014)) was introduced either upstream in sense orientation or downstream in antisense orientation for inducible expression or downregulation, respectively.
Single mutants for validation of hits important for survival at exponential phase were grown in the presence of 1 mm IPTG until OD600 of 0.1. Then 108 CFU were subjected to static incubation in LB or fresh water, or LB supplemented with gentamicin incubation with shaking for 15 min. For validation of stationary phase hits, individual mutants were grown for 16 h at 37 °C with aeration. The next day, cultures were mixed in a 1:1 ratio with E7946ΔlacZ and 108 CFU were challenged with 2 ml of fresh water or LB broth. Survival of individual mutants was assayed by serial dilution and plating for viable CFU on LB agar plates supplemented with X-Gal for blue/white screening. The competitive index (CI) was calculated as: CFU of mutant/CFU of ΔlacZ wild-type corrected for any deviation from a ratio of 1 in the input.
Infant mice and infant rabbit colonization experiments
5-day-old litters of infant CD1 mice were inoculated orogastrically with 50 μl containing ~105 CFU of the wild-type or a mutant strain of V. cholerae as described (Tischler and Camilli, 2005). After 24 h, mice were euthanized, the small intestine was removed and homogenized in 1 ml of LB broth supplemented with 20% glycerol. Colonization was assayed by serial dilution and plating for viable CFU on LB agar plates supplemented with Sm100 (Camilli et al., 1994; Schild et al., 2007). 2- to 3-day old infant rabbits were infected as described (Kamp et al., 2013). V. cholerae was recovered from the cecal lumenal fluid and colonization levels were assayed by serial dilution and plating for viable CFU as described above for infant mice outputs. All animal procedures were conducted in accordance with the rules of the Department of Laboratory Animal Medicine at Tufts Medical Center.
Results
Viability of V. cholerae upon exposure to fresh water is growth phase dependent
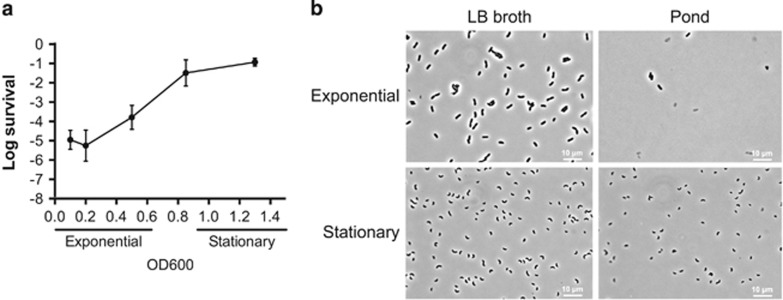
As the ~50-fold drop in conductivity from rice-water stool is the same as from Luria Bertani (LB) broth (conductivity: 19.4 mS cm−2) to fresh (pond) water (conductivity: 280 μS cm2) (Nelson et al., 2008), we determined the survival rate of V. cholerae when transferred from LB broth to fresh water collected from the Massapoag Lake in MA, USA. Consistent with a previous report (Kamp et al., 2013), we observed a strong correlation between the physiological state of growth and survival wherein V. cholerae grown to stationary phase survived in fresh water (Figure 1a). In the previous study (Kamp et al., 2013), long-term survival was examined, whereas here we examine short-term (15 min) survival to hypo-osmotic shock. This time point was sufficient to observe killing by osmotic shock without inducing the stringent response due to the nutrient-poor environment. We observed a positive correlation between survival and progression through the growth curve as cells approached stationary phase (Figure 1a). The number of visibly intact bacteria was greatly reduced when rapidly growing bacteria were exposed to fresh water (Figure 1b). Hence, lysis appears to be the dominant mechanism for the loss in viability. Consistent with fresh water inducing osmotic shock, a 5-fold dilution of the chemically defined growth medium M9 was sufficient to achieve the same level of killing (conductivity: 2.5 mS cm−2) (Supplementary Figure 1A). Moreover, the addition of NaCl or the non-metabolizable osmolyte, polyethylene glycol of molecular weight 3350 (PEG-3350) to fresh water resulted in full protection (Supplementary Figure 1B). These data support the hypothesis that the initial loss of viability observed upon fresh water challenge is due primarily if not entirely to osmotic shock and that rapidly growing bacteria are most susceptible to this shock.
Figure 1.
Growth-dependent survival of V. cholerae to fresh water challenge. Cultures of V. cholerae E7946 were grown with aeration at 37 °C in LB broth to an OD600 of 0.1 (exponential phase), 0.5 (exponential phase), 0.8 (early stationary phase) and 1.3 (stationary phase). 1 ml aliquots were washed and incubated for 15 min in fresh water and survival was measured (a). Phase contrast microscopic images of exponential (log) and stationary phase bacteria before and after fresh water challenge (b).
Transposon sequencing screens identify pathways involved in osmotic shock survival
We examined the genes involved in the susceptibility of rapidly growing cells to osmotic shock. Three mini-Tn10 (mTn10) insertion libraries were made, each of ~60 000 complexity. The mTn10 derivatives each contain one outwardly transcribing, IPTG-inducible promoter (Ptac) and genes conferring resistance to either Spectinomycin, Kanamycin or Ampicillin. The libraries were grown in LB broth supplemented with 1 mm IPTG to early exponential phase and challenged with fresh water. The Ptac enabled overexpression of downstream genes oriented in the same direction or inhibition of expression of genes oriented in the antisense direction (McDonough et al., 2014).
High-throughput transposon sequencing (Tn-seq) (van Opijnen et al., 2009), was used to identify insertion mutants with improved survival. These fell into several functional classes including; perturbed extra-cytosolic proteins, decreased DNA repair and replication functions, decreased sigma factor RpoN and nitrogen metabolism, or decreased tRNA charging and repair. Among the last category, we found that the survivor population after osmotic shock was enriched in mTn10 insertions downstream of almost all tRNA synthetase genes whereby Ptac was positioned in the antisense orientation. In contrast, in the input pool, transposon insertions were not enriched downstream of tRNA synthetase genes and those that were present were found equally in either orientation (Supplementary Tables 1 and 4). We hypothesize that antisense expression from Ptac lowered tRNA synthetase activity and this enabled survival following osmotic shock treatment. Inhibition of tRNA synthetase activity can cause ribosome stalling and increased production of the stringent response alarmone (p)ppGpp. Consistent with this, we also found enriched mTn10 insertions that are hypothesized to increase (p)ppGpp directly through increased synthesis or reduced degradation, or indirectly through increased expression of a hipAB-like toxin–antitoxin system. These results are consistent with the idea that V. cholerae can survive osmotic shock by slowing down different metabolic processes thereby enabling dormancy. Interestingly, many of the same genetic alterations found here to enhance resistance to osmotic shock such as those related to the stringent response have also been shown to enhance survival upon exposure to antibiotics (Germain et al., 2013; Kaspy et al., 2013). So-called persister cells are similarly associated with the slowing of metabolic processes and partial or complete dormancy.
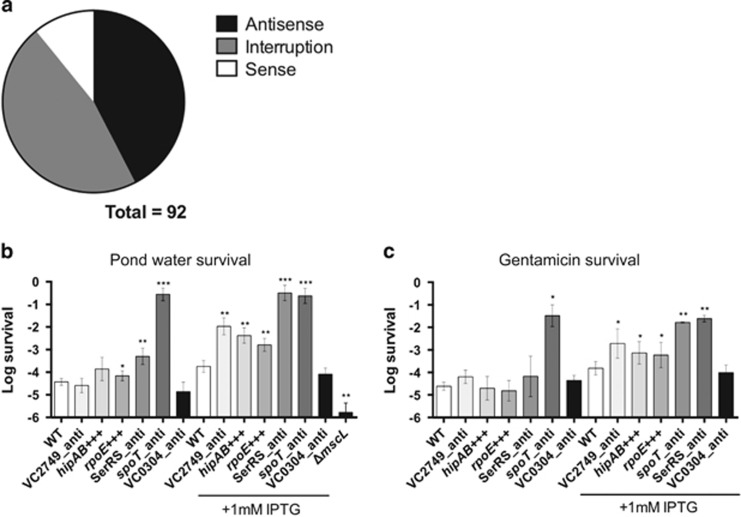
In this gain of function screen, 53% of the mTn10 insertions that led to improved osmotic resistance functioned in an orientation dependent manner by virtue of their outwardly transcribing Ptac (Figure 2a). Via both sense and antisense mechanisms, the expression of adjacent genes was modulated. This enabled the detection of effects conferred by essential genes that, by definition, could not be observed through insertional inactivation.
Figure 2.
Mutants with increased resistance to hypo-osmotic shock following rapid aerobic growth. Classification of directionality of mTn10 insertions found in the survivors to osmotic shock population (a). Cultures of V. cholerae E7946 and the derivatives shown below the x-axis were grown with aeration at 37 °C in LB broth with or without IPTG to an OD600 of 0.1. 1 ml aliquots were washed and incubated for 15 min in fresh water (b, upper panel) or LB broth plus gentamicin (c, down panel). Bars represent the average of at least four independent assays. Error bars show standard error. Significance was calculated using Student’s two-tailed t-test (*<0.05, **<0.01).
To validate the results from our screen, we introduced a Ptac with an associated lacI repressor by site-specific integration into the same location in the genome as was observed for particular mTn10 mutants (Supplementary Table 2). As homologs of many of the genes identified here as important for osmotic shock survival have also been linked to persistence to antibiotic stress in other bacteria, we compared the rate of survival of these mutants when challenged with either fresh water or gentamicin. A deletion of the mechanosensitive channel gene mscL, believed to be important in maintaining osmotic balance (Nakamaru et al., 1999; Booth and Blount, 2012; Rowe et al., 2013), reduced the survival of V. cholerae to osmotic shock (Figure 2b). In contrast, both an increased resistance to osmotic shock and to gentamicin challenge was observed upon antisense depletion of a seryl-tRNA synthetase, SerRS, and a (p)ppGpp hydrolase, spoT, and with the overexpression of the hipAB toxin–antitoxin system. Similarly, antisense depletion of a nitrogen regulation protein (VC2749), and overexpression of the sigma factor RpoE operon enhanced survival to challenge with both fresh water and gentamicin. In general, each of the mutants tested showed a similar degree of increased survival with each of the two very different challenges (Figures 2b and c). All mutants capable of increasing resistance upon IPTG induction showed an accompanying decrease in their growth rates. Thus, we hypothesize that in a rapidly growing population of wild-type V. cholerae, the slow or non-growing persister subpopulation is resistant to both fresh water and gentamicin challenges while the remaining cells are sensitive to each challenge.
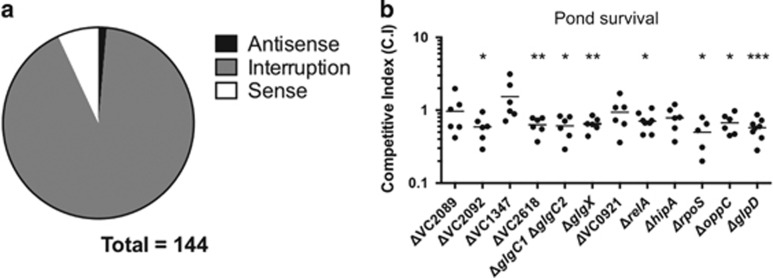
To search for mutants that exhibit decreased survival after osmotic shock in fresh water, we screened mTn10 insertion libraries after growth to stationary phase in which the survival of V. cholerae decreases by only ~50%. In contrast to the previous screen, here we almost exclusively identified mutants that interrupted genes independent of transposon orientation (Figure 3a). Mutants with increased sensitivity to fresh water mapped primarily to genes involved in the maintenance of the redox-state (TCA cycle, oxidative stress response) or to membrane transporters. Previous studies observed that disruption of efflux pumps and respiration-related genes showed a decreased level of persister cells in E. coli (Orman and Brynildsen, 2015; Pu et al., 2016). Hence, again we observed an at least partial overlap between the genes involved in osmotic stress in V. cholerae and those involved in antibiotic persistence in other species. As expected, we also found genes not involved in persistence but rather involved in the production of osmoprotectants like glycogen, l-methionine and l-glutamate (Bougouffa et al., 2014; Supplementary Tables 3 and 5).
Figure 3.
Mutants grown to stationary phase with reduced resistance to hypo-osmotic shock. Classification of directionality of mTn10 insertions found in the mutants with decreased survival to osmotic shock by fresh water (a). V. cholerae E7946ΔlacZ was subjected to competition assays in a 1:1 ratio with its derivative mutants. Approximately 108 CFU were washed and incubated for 15 min in fresh water. Graph represent the average of at least six independent assays (b). Graphs represent the average of at least six independent assays. Error bars show standard error. Significance was calculated using Student’s two-tailed t-test (*<0.05, **<0.01, ***<0.001).
To validate specific candidates identified in this screen, we tested deletion mutants belonging to the categories identified and subjected them to osmotic shock in a 1:1 ratio in competition with the wild-type strain. Mutants involved in respiration, glycogen metabolism, l-glutamate generation and oligopeptide transport showed decreased survival to osmotic shock by 2-3-fold. Also, the ΔrelA, ΔrpoS and ΔglpD mutants which have been previously linked to persistence (Spoering et al., 2006; Amato et al., 2013; Radzikowski et al., 2016) showed a decreased survival to osmotic shock (Figure 3b). It is likely that, due to the redundancy of survival mechanisms that oppose osmotic stress during stationary phase, we were only able to find mild phenotypes when single mutants were tested.
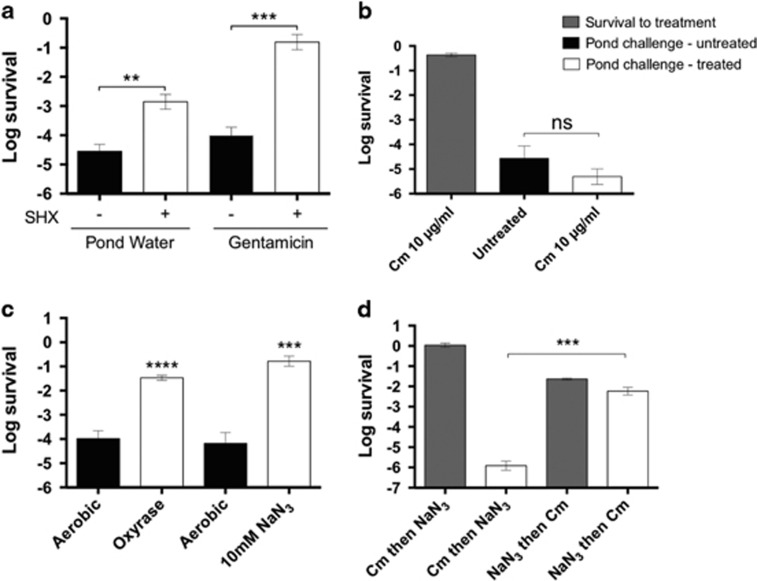
To further evaluate the role of (p)ppGpp and the stringent response in the survival to hypo-osmotic shock, we generated a ΔrelA ΔrelV ΔspoT (p)ppGpp0 strain as previously described (Das et al., 2009). An exponentially growing culture of the (p)ppGpp0 strain had no discernable defect in surviving osmotic shock or gentamicin treatment. However, a stationary culture of the same strain had an ~8-fold defect for both conditions (Supplementary Figure 2A). We next tested the effect of increasing (p)ppGpp level in rapidly growing cells on survival following osmotic shock. Addition of a serine analog (DL-serine hydroxamate or SHX) is commonly used to increase both (p)ppGpp level and to induce the persister state in other bacteria (Tosa and Pizer, 1971). In V. cholerae, SHX treatment stimulated fresh water survival by nearly 100-fold and stimulated gentamicin persistence by roughly 1000-fold (Figure 4a). In summary, (p)ppGpp has a significant role in the survival of stationary phase cells following osmotic shock but is not required for the survival of exponential phase cells. However, when (p)ppGpp level is artificially increased in exponential phase cells, survival is greatly enhanced.
Figure 4.
Induction of the stringent response or inhibition of respiration protects rapidly growing cultures from hypo-osmotic shock via a mechanism that requires de novo protein synthesis. Survival of exponential V. cholerae E7946 cultures in the presence of 1 mg ml−1 of SHX (a) or Cm 10 μg ml−1 (b) before challenge by osmotic shock or gentamicin. Alternatively, cultures were treated with Oxyrase or sodium azide (NaN3) before osmotic shock challenge (c). Effect on the order of addition between chloramphenicol and NaN3 evaluated in fresh water challenge (d). Graphs represent the average of at least three independent assays. Error bars show standard error. Significance was calculated using Student’s two-tailed t-test (**<0.01, ***<0.001, ****<0.0001).
We next examined the mechanism(s) that enable stationary phase V. cholerae to survive exposure to fresh water. Quorum sensing molecules are produced and accumulate to high extracellular concentrations as cells enter stationary phase and cause profound changes in gene expression (Goo et al., 2012). We therefore tested whether these molecules could induce a genetic program capable of protecting cells from osmotic shock. V. cholerae was grown to exponential phase in spent medium from an overnight culture that was supplemented with fresh nutrients. We observed that bacteria grown either in the presence or absence of spent medium were equally susceptible to fresh water (Supplementary Figure 2B). This suggests that the high resistance to osmotic shock seen with stationary phase bacteria is not due to the action of quorum sensing molecules. Rather, it likely results from either a reduction in cell division and metabolic activities and/or from the induction of unknown mechanisms. However, we cannot completely rule out a possible role of quorum sensing in this process: (i) there is a possibility that QS-regulated genes are not induced in this physiological state, even when autoinducers were added and (ii) autoinducers could be unstable and could have been degraded in the spent medium. Further experiments would be needed to fully understand the implications of quorum sensing in osmotic shock resistance.
To test whether stopping cell division and metabolic activities alone is sufficient to protect from osmotic shock, we added chloramphenicol to exponentially growing V. cholerae cultures for 30 min prior to the fresh water challenge. This treatment both prevents de novo protein synthesis and causes a complete but reversible arrest of growth (Wisseman et al., 1954). Although cells survived chloramphenicol treatment in the absence of fresh water challenge, chloramphenicol treatment did not increase resistance to osmotic shock (Figure 3b). We conclude that cessation of growth and metabolic activity is insufficient to protect against osmotic shock. Furthermore, our results suggest that the protection observed upon transition to stationary phase requires de novo protein synthesis.
As mentioned earlier, respiration has also been linked to the generation of persister cells (Orman and Brynildsen, 2015). We tested the effect of oxygen availability on osmotic shock survival by incubating the cultures statically in the presence of Oxyrase (Oxyrase, Inc) to produce a microaerobic condition. Strikingly, exponential cultures grown in this condition showed >100-fold improved survival (Figure 4c). In contrast, stationary phase cultures grown in this condition exhibited no change in viability upon treatment with fresh water (Supplementary Figure 3A).
To further test the role of respiration in osmotic shock survival, exponential and stationary phase cultures were exposed to the respiration inhibitor sodium azide (NaN3). Addition of NaN3 to stationary phase cultures had no effect on viability upon exposure to fresh water (Supplementary Figure 3B). In contrast, addition of NaN3 to exponential cultures resulted in a 1000-fold increase in survival (Figure 4c). Importantly, the growth rate of cells both in the microaerobic culture and when treated with NaN3 was greatly diminished. NaN3 is not only an inhibitor of the electron transport chain but has also been observed to the inhibit DNA synthesis and cell division (Donachie, 1969; Cieśla et al., 1974; Fortin et al., 1990). These data show that slowing or stopping growth of V. cholerae by inhibiting respiration greatly increases resistance to osmotic shock.
It is possible that upon transitioning from rapid aerobic growth to that in the presence of NaN3, a response is triggered that includes the synthesis of new proteins that directly or indirectly protect against osmotic shock. To test this possibility, we did an order of addition experiment that used both chloramphenicol and NaN3. In one case, exponentially growing cultures were treated with chloramphenicol for 30 min followed by treatment with NaN3 for 90 min prior to the fresh water challenge. If NaN3 treatment rescues cells from osmotic shock by inducing the synthesis of protective proteins then, a prior chloramphenicol treatment should block rescue. As a control for this experiment, the order of addition was reversed. In this case, there is a 90-min window where NaN3 can still induce new protein synthesis prior to the addition of chloramphenicol. Treatment with chloramphenicol before but not after NaN3 treatment blocked rescue from osmotic lysis (Figure 4d). Thus, we conclude that treatment with NaN3 induces a response that triggers the production of new proteins which protect against osmotic shock.
Persisters are formed during infection thereby facilitating dissemination of V. cholerae and a V. cholerae mutant strain with a higher proportion of persisters is more efficient at colonizing the small intestine
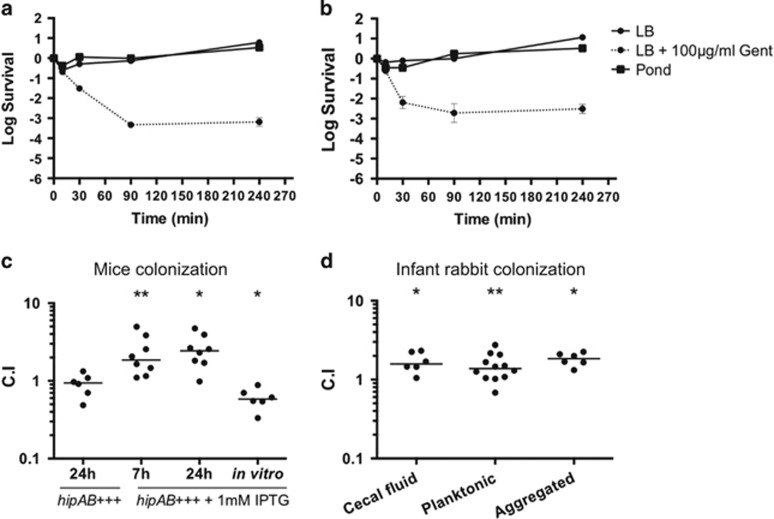
To evaluate persister formation during infection, the planktonic and aggregated V. cholerae from infected infant rabbit cecal fluid samples were separated by differential centrifugation and exposed to LB broth, fresh water or gentamicin (Supplementary Figure 4A). Just before challenge, aggregates of V. cholerae were disrupted by vigorous mechanical resuspension (Supplementary Figure 4B) so that accurate titers could be measured. Consistent with a previous report (Kamp et al., 2013), V. cholerae from cecal fluid is highly resistant to osmotic shock (Figure 5a, squares). To determine the presence of persisters, ~108 CFU of V. cholerae from either aggregated or planktonic populations were incubated in the presence of 15-times the minimal inhibitory concentration (MIC) of gentamicin for up to 4 h with aeration at 25 °C. After antibiotic treatment, we were able to isolate V. cholerae persisters from both portions of the cecal fluid (Figures 5a and b, circles with dashed lines).
Figure 5.
Persister-like subpopulations of V. cholerae exist during infection of the infant rabbit model and a V. cholerae mutant with a higher proportion of persisters outcompetes the wild-type strain in animal models of infection. 108 CFU of planktonic (a) or disrupted aggregates (b) of V. cholerae from cecal fluid were incubated in LB (circles), Fresh water (squares) or LB supplemented with 100 μg ml−1 of gentamicin (15X MIC, circles dashed lines). Aliquots were taken at 15, 90 or 240 min and viability was measured by serial dilution and plating on Sm100 plates. CD1 mice were inoculated with a 1:1 mixture of an induced or non-induced expression of the toxin–antitoxin system hipAB-like and the wild-type strain for competition assays and assessment of colonization abilities of small intestine in the infant mouse (c) or cecal fluid in the infant rabbit model (d). Significance was calculated using Student’s two-tailed t-test (*<0.05, **<0.01).
Since our data suggests that at least a portion of V. cholerae shed to the environment are in a persister state, we sought to study the importance of V. cholerae persister formation with respect to infectivity. We hypothesize that environmental V. cholerae in a persister state could impact transmission of this pathogen by lowering the dose needed to cause a successful infection, since they would be more resilient to stress such as the acidic pH in the stomach. Then, as soon as the stress is no longer present, persisters would leave this dormant state and start replicating, leading to a successful infection. To evaluate this, we used a hipAB+++ strain that induces the production of a toxin–antitoxin module and therefore a persister-like state by addition of IPTG, and compared its ability to colonize infant mice with that of the non-induced and wild-type parental strains. Groups of 5-day-old mice were inoculated with an induced or non-induced hipAB+++ strain, each mixed 1:1 with the wild-type strain for competition assays. Only the induced hipAB+++ strain was able to outcompete the wild-type strain by ~2-fold (Figure 5c). This difference is highlighted by the fact that this strain was out-competed by the wild-type strain by ~2-fold when grown in vitro (Figure 5c). Thus, even though the hipAB+++ strain grows slightly slower than the wild-type strain, it was still able to outcompete wild-type V. cholerae during infection.
We next examined the importance of persister formation in the infection of infant rabbits, which, in contrast to infant mice, develop profuse secretory diarrhea akin to human cholera (Ritchie et al., 2010; Kamp et al., 2013; Abel and Waldor, 2015). As in human rice-water stool (Nelson et al., 2007), cecal fluid obtained from symptomatic infant rabbits show two distinctive populations, highly-motile (planktonic) and aggregated V. cholerae. We induced the persister state in the hipAB+++ strain and, in competition with the wild-type strain, assayed its ability to colonize. As seen in the infant mouse model, the induced hipAB+++ out-competed the wild-type strain by ~2-fold in both the planktonic and aggregated subpopulations (Figure 5d).
Discussion
Human shed V. cholerae experience a dramatic change in osmolarity and nutrient availability when transmitted to fresh water environments, yet its success as a water-born pathogen requires that it survives this stressful transition. Several studies have addressed osmotic challenges to V. cholerae survival, however, most of these have focused on transition to high salinity environments (Chakrabarti et al., 1996; Häse and Mekalanos, 1999; Pflughoeft et al., 2003; Kapfhammer et al., 2005; Nag et al., 2005; Shikuma and Yildiz, 2009; Jahid et al., 2013; Rowe et al., 2013; Shikuma et al., 2013; Möll et al., 2015). Since, during outbreaks and epidemics, V. cholerae is consumed in contaminated drinking water, its ability to withstand low salinity conditions is critical to this mode of transmission. In this study, we provide insights into the mechanisms of tolerance to a low osmotic aquatic environment and identified stationary and persistent V. cholerae subpopulations that show vastly improved rates of survival under these conditions.
The ability of V. cholerae to form persister subpopulations has previously been suggested (Miller et al., 1984; Jubair et al., 2012; 2014) but not rigorously shown, nor have the mechanisms involved in persister formation been examined in this organism. Here we formally show persister formation in V. cholerae and its relevance in both the pathogenic and environmental stages of its life cycle. We determined that V. cholerae from both in vitro cultures and animal infections include a subpopulation of persister-like bacteria that is more resistant to antibiotic treatment and to osmotic shock.
The most common class of mutants with improved survival identified in our Tn-Seq screen were those associated with the stringent response and (p)ppGpp metabolism. (p)ppGpp is produced by the dominant synthase, RelA, under starvation conditions, where the ratio of charged/uncharged tRNA is low (reviewed in Potrykus and Cashel, 2008). Of the 22 annotated tRNA synthestases in the V. cholerae genome, 19 were found as over-represented in the output pool in association with downstream mTn10 insertions that were inserted in the antisense orientation. As all 19 genes are essential, the downstream antisense insertions provided a non-lethal way to lower synthetase activity thereby increasing the uncharged tRNA pool and (p)ppGpp production. Consistent with this, we found that a chemical inhibitor of a serine tRNA synthestase, SHX, which is known to induce (p)ppGpp in other organisms, also similarly induced enhanced resistance to hypo-osmotic shock in V. cholerae. Finally, other mutants found in the screen that are expected to cause increased levels of (p)ppGpp included antisense insertions downstream of the dominant (p)ppGpp hydrolase, SpoT, and insertions oriented to overexpress the the HipAB toxin–antitoxin system. These findings are in agreement with a previous report in which a ppGpp-dependent response to hyperosmotic shock was found in E. coli (Tarusawa et al., 2016), indicating that (p)ppGpp would be a conserved mechanism to respond to osmotic stress.
Essentially all the mutants that we identified to have increased survival to fresh water challenge, including those involved in the stringent response, displayed slower growth rates and/or reduced metabolism. Experiments with chloramphenicol suggested that reduced metabolic activity and growth rate alone are insufficient to promote survival to low osmotic transitions. Rather, de novo protein synthesis is required. It is important to note that chloramphenicol inhibits translation in a manner that prevents (p)ppGpp formation (Sokawa and Sokawa, 1978). It is therefore likely that the proteins produced during the transition to stationary phase that protect against osmotic shock are at least partially induced by the stringent response.
We also found that upregulation of RpoE was an alternate mechanism responsible for improved survival. Consistent with a role in osmoprotection, this sigma factor is involved in the expression of genes that respond to stresses affecting the cell wall, envelopes and periplasm (Moreau, 2014). Downregulation of RpoN, a sigma factor that competes with RpoE for RNA polymerase (Merrick, 1993), also conferred better survival to osmotic shock. Such downregulation is expected to decrease glutamine and arginine biosynthesis (Kloosterman et al., 2006), leading to the accumulation of glutamate which can be used as a compatible solute and osmoprotectant (Goude et al., 2004; Bougouffa et al., 2014; Shao et al., 2015). Accumulation of glycogen also proved to be useful as an osmoprotectant in our assays. This is of special importance since glycogen might have a dual role as osmprotectant and carbon source storage for survival in the aquatic environment as has been described (Bourassa and Camilli, 2009).
In summary, when exposed to an extreme and immediate osmotic downshift, rapidly growing V. cholerae are unable to mount an effective response. However, those cells already in the persister state, in stationary phase, or that had experienced reduced growth due to oxygen limitation can withstand the initial shock and go on to disseminate. Since the vast majority of V. cholerae cells shed in rice-water stool or from animal model infections are resistant to fresh water challenge, it is likely that, late in infection, there are very few cells experiencing rapid growth. This is consistent with a report that rice-water stool is microaerobic and unable to support growth of V. cholerae (Freter et al., 1961).
This is the first study to identify the presence of V. cholerae persisters during infection. In other bacteria, persisters enable survival in a host during treatment with antibiotics and are responsible for relapses that occur following the cessation of treatment. The excessive use of antibiotics in many of the regions endemic for V. cholerae is well documented and is thought to be responsible for the emergence and dominance of strains resistant to multiple antibiotics (Beaber et al., 2002). In this context, it is likely that the ability of V. cholerae to form persisters during infection aids its survival in humans taking antibiotics.
In addition to any potential role in survival during antibiotic treatment, we propose that the generation of dormant and persister subpopulations during V. cholerae infections ensures survival following exit from the host and its subsequent exposure to low osmotic, nutrient-poor environments. Further experiments will be needed to determine whether such subpopulations are also important for survival in estuarine and salt water environments and/or in other aspects of the V. cholerae life cycle.
Acknowledgments
We thank the members of the AC lab for helpful discussions. This work was supported by NIH grant AI055058 to AC. CASV was supported by the Pew Latin American Fellows Program in the Biomedical Sciences and a CONICYT Becas Chile postdoctoral fellowship. AC is an investigator with the Howard Hughes Medical Institute.
Footnotes
Supplementary Information accompanies this paper on The ISME Journal website (http://www.nature.com/ismej)
The authors declare no conflict of interest.
Supplementary Material
References
- Abel S, Waldor MK. (2015). Infant rabbit model for diarrheal diseases. Curr Protoc Microbiol 38: 6A.6.1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amato SM, Orman MA, Brynildsen MP. (2013). Metabolic control of persister formation in Escherichia coli. Moll Cell 50: 475–487. [DOI] [PubMed] [Google Scholar]
- Beaber JW, Burrus V, Hochhut B, Waldor MK. (2002). Comparison of SXT and R391, two conjugative integrating elements: definition of a genetic backbone for the mobilization of resistance determinants. Cell Mol Life Sci 59: 2065–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth IR, Blount P. (2012). The MscS and MscL families of mechanosensitive channels act as microbial emergency release valves. J Bacteriol 194: 4802–4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bougouffa S, Radovanovic A, Essack M, Bajic VB. (2014). DEOP: a database on osmoprotectants and associated pathways. Database (Oxford) 2014: pii: bau100. [DOI] [PMC free article] [PubMed]
- Bourassa L, Camilli A. (2009). Glycogen contributes to the environmental persistence and transmission of Vibrio cholerae. Mol Microbiol 72: 124–138.19226328 [Google Scholar]
- Camilli A, Beattie DT, Mekalanos JJ. (1994). Use of genetic recombination as a reporter of gene expression. Proc Natl Acad Sci USA 91: 2634–2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti SR, Chaudhuri K, Sen K, Das J. (1996). Porins of Vibrio cholerae: purification and characterization of OmpU. J Bacteriol 178: 524–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieśla Z, Mardarowicz K, Klopotowski T. (1974). Inhibition of DNA synthesis and cell division in Salmonella typhimurium by azide. Mol Gen Genet 135: 339–348. [DOI] [PubMed] [Google Scholar]
- Colwell RR. (2000). Viable but nonculturable bacteria: a survival strategy. J Infect Chemother 6: 121–125. [DOI] [PubMed] [Google Scholar]
- Dalia AB, Lazinski DW, Camilli A. (2014). Identification of a membrane-bound transcriptional regulator that links chitin and natural competence in Vibrio cholerae. mBio 5: e01028–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das B, Pal RR, Bag S, Bhadra RK. (2009). Stringent response in Vibrio cholerae: genetic analysis of spoT gene function and identification of a novel (p)ppGpp synthetase gene. Mol Microbiol 72: 380–398. [DOI] [PubMed] [Google Scholar]
- Donachie WD. (1969). Control of cell division in Escherichia coli: experiments with thymine starvation. J Bacteriol 100: 260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin Y, Phoenix P, Drapeau GR. (1990). Mutations conferring resistance to azide in Escherichia coli occur primarily in the secA gene. J Bacteriol 172: 6607–6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R, Smith HL, Sweeney FJ. (1961). An evaluation of intestinal fluids in the pathogenesis of cholera. J Infect Dis 109: 35–42. [DOI] [PubMed] [Google Scholar]
- Germain E, Castro-Roa D, Zenkin N, Gerdes K. (2013). Molecular mechanism of bacterial persistence by HipA. Mol Cell 52: 248–254. [DOI] [PubMed] [Google Scholar]
- Goo E, Majerczyk CD, An JH, Chandler JR, Seo Y-S, Ham H et al. (2012). Bacterial quorum sensing, cooperativity, and anticipation of stationary-phase stress. Proc Natl Acad Sci USA 109: 19775–19780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goude R, Renaud S, Bonnassie S, Bernard T, Blanco C. (2004). Glutamine, glutamate, and alpha-glucosylglycerate are the major osmotic solutes accumulated by Erwinia chrysanthemi strain 3937. Appl Environ Microbiol 70: 6535–6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häse CC, Mekalanos JJ. (1999). Effects of changes in membrane sodium flux on virulence gene expression in Vibrio cholerae. Proc Natl Acad Sci USA 96: 3183–3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton RM, Hunt HD, Ho SN, Pullen JK, Pease LR. (1989). Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene 77: 61–68. [DOI] [PubMed] [Google Scholar]
- Jahid IK, Hasan MM, Abdul Matin M, Mahmud ZH, Neogi SB, Uddin MH et al. (2013). Role of polyphosphate kinase gene (ppk) for survival of Vibrio cholerae O1 in surface water of Bangladesh. Pak J Biol Sci 16: 1531–1537. [DOI] [PubMed] [Google Scholar]
- Johnson CN. (2013). Fitness factors in Vibrios: a mini-review. Microb Ecol 65: 826–851. [DOI] [PubMed] [Google Scholar]
- Jubair M, Atanasova KR, Rahman M, Klose KE, Yasmin M, Yilmaz Ö et al. (2014). Vibrio cholerae persisted in microcosm for 700 days inhibits motility but promotes biofilm formation in nutrient-poor lake water microcosms. PLoS ONE 9: e92883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jubair M, Morris JG, Ali A. (2012). Survival of Vibrio cholerae in nutrient-poor environments is associated with a novel ‘persister’ phenotype. PLoS One 7: e45187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamp HD, Patimalla-Dipali B, Lazinski DW, Wallace-Gadsden F, Camilli A. (2013). Gene fitness landscapes of Vibrio cholerae at important stages of its life cycle. Plos Pathog 9: e1003800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapfhammer D, Karatan E, Pflughoeft KJ, Watnick PI. (2005). Role for glycine betaine transport in Vibrio cholerae osmoadaptation and biofilm formation within microbial communities. Appl Environ Microbiol 71: 3840–3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspy I, Rotem E, Weiss N, Ronin I, Balaban NQ, Glaser G. (2013). HipA-mediated antibiotic persistence via phosphorylation of the glutamyl-tRNA-synthetase. Nat commun 4: 1–7. [DOI] [PubMed] [Google Scholar]
- Kell DB, Kaprelyants AS, Weichart DH, Harwood CR, Barer MR. (1998). Viability and activity in readily culturable bacteria: a review and discussion of the practical issues. Antonie Van Leeuwenhoek 73: 169–187. [DOI] [PubMed] [Google Scholar]
- Kloosterman TG, Hendriksen WT, Bijlsma JJE, Bootsma HJ, van Hijum SAFT, Kok J et al. (2006). Regulation of glutamine and glutamate metabolism by GlnR and GlnA in Streptococcus pneumoniae. J Biol Chem 281: 25097–25109. [DOI] [PubMed] [Google Scholar]
- Lazinski DW, Camilli A. (2013). Homopolymer tail-mediated ligation PCR: a streamlined and highly efficient method for DNA cloning and library construction. Biotechniques 54: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine MM, Black RE, Clements ML, Cisneros L, Saah A, Nalin DR et al. (1982). The pathogenicity of nonenterotoxigenic Vibrio cholerae serogroup O1 biotype El Tor isolated from sewage water in Brazil. J Infect Dis 145: 296–299. [DOI] [PubMed] [Google Scholar]
- McDonough E, Lazinski DW, Camilli A. (2014). Identification of in vivo regulators of the Vibrio cholerae xds gene using a high-throughput genetic selection. Mol Microbiol 92: 302–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick MJ. (1993). In a class of its own — the RNA polymerase sigma factor σ54 (σN). Mol Microbiol 10: 903–909. [DOI] [PubMed] [Google Scholar]
- Miller CJ, Drasar BS, Feachem RG. (1984). Response of toxigenic Vibrio cholerae 01 to physico-chemical stresses in aquatic environments. J Hyg 93: 475–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau PL. (2014). Protective role of the RpoE (σE) and Cpx envelope stress responses against gentamicin killing of nongrowing Escherichia coli incubated under aerobic, phosphate starvation conditions. FEMS Microbiol Lett 357: 151–156. [DOI] [PubMed] [Google Scholar]
- Möll A, Dörr T, Alvarez L, Davis BM, Cava F, Waldor MK. (2015). A D, D-carboxypeptidase is required for Vibrio cholerae halotolerance. Environ Microbiol 17: 527–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nag S, Das S, Chaudhuri K. (2005). In vivo induced clpB1 gene of Vibrio cholerae is involved in different stress responses and affects in vivo cholera toxin production. Biochem Biophys Res Commun 331: 1365–1373. [DOI] [PubMed] [Google Scholar]
- Nakamaru Y, Takahashi Y, Unemoto T, Nakamura T. (1999). Mechanosensitive channel functions to alleviate the cell lysis of marine bacterium, Vibrio alginolyticus, by osmotic downshock. FEBS Lett 444: 170–172. [DOI] [PubMed] [Google Scholar]
- Nelson EJ, Chowdhury A, Flynn J, Schild S, Bourassa L, Shao Y et al. (2008). Transmission of Vibrio cholerae is antagonized by lytic phage and entry into the aquatic environment. PLoS Pathog 4: e1000187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EJ, Chowdhury A, Harris JB, Begum YA, Chowdhury F, Khan AI et al. (2007). Complexity of rice-water stool from patients with Vibrio cholerae plays a role in the transmission of infectious diarrhea. Proc Natl Acad Sci USA 104: 19091–19096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orman MA, Brynildsen MP. (2015). Inhibition of stationary phase respiration impairs persister formation in E. coli. Nat Commun 6: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflughoeft KJ, Kierek K, Watnick PI. (2003). Role of ectoine in Vibrio cholerae osmoadaptation. Appl Environ Microbiol 69: 5919–5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potrykus K, Cashel M. (2008). (p)ppGpp: still magical? Annu Rev Microbiol 62: 35–51. [DOI] [PubMed] [Google Scholar]
- Pu Y, Zhao Z, Li Y, Zou J, Ma Q, Zhao Y et al. (2016). Enhanced efflux activity facilitates drug tolerance in dormant bacterial cells. Mol Cell 62: 284–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radzikowski JL, Vedelaar S, Siegel D, Ortega ÁD, Schmidt A, Heinemann M. (2016). Bacterial persistence is an active σS stress response to metabolic flux limitation. Mol Syst Biol 12: 882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie JM, Rui H, Bronson RT, Waldor MK. (2010). Back to the future: studying cholera pathogenesis using infant rabbits. mBio 1: e00047–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe I, Elahi M, Huq A, Sukharev S. (2013). The mechanoelectrical response of the cytoplasmic membrane of Vibrio cholerae. J Gen Physiol 142: 75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schild S, Tamayo R, Nelson EJ, Qadri F, Calderwood SB, Camilli A. (2007). Genes induced late in infection increase fitness of Vibrio cholerae after release into the environment. Cell Host Microbe 2: 264–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Z, Deng W, Li S, He J, Ren S, Huang W et al. (2015). GlnR-mediated regulation of ectABCD transcription expands the role of the GlnR regulon to osmotic stress management. J Bacteriol 197: 3041–3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikuma NJ, Davis KR, Fong JNC, Yildiz FH. (2013). The transcriptional regulator, CosR, controls compatible solute biosynthesis and transport, motility and biofilm formation in Vibrio cholerae. Environ Microbiol 15: 1387–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikuma NJ, Yildiz FH. (2009). Identification and characterization of OscR, a transcriptional regulator involved in osmolarity adaptation in Vibrio cholerae. J Bacteriol 191: 4082–4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokawa J, Sokawa Y. (1978). Relaxation effect of chloramphenicol on the stringent control in Escherichia coli. J Biochem 83: 1699–1705. [DOI] [PubMed] [Google Scholar]
- Spoering AL, Vulic M, Lewis K. (2006). GlpD and PlsB participate in persister cell formation in Escherichia coli. J Bacteriol 188: 5136–5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarusawa T, Ito S, Goto S, Ushida C, Muto A, Himeno H. (2016). (p)ppGpp-dependent and -independent pathways for salt tolerance in Escherichia coli. J Biochem 160: 19–26. [DOI] [PubMed] [Google Scholar]
- Tischler AD, Camilli A. (2005). Cyclic diguanylate regulates Vibrio cholerae virulence gene expression. Infect Immun 73: 5873–5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosa T, Pizer LI. (1971). Biochemical bases for the antimetabolite action of L-serine hydroxamate. J Bacteriol 106: 972–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Opijnen T, Bodi KL, Camilli A. (2009). Tn-seq: high-throughput parallel sequencing for fitness and genetic interaction studies in microorganisms. Nat Methods 6: 767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisseman CL Jr, Smadel JE, Hahn FE, Hopps HE. (1954). Mode of action of chloramphenicol I : action of chloramphenicol on assimilation of ammonia and on synthesis of proteins and nucleic acids in Escherichia coli. J Bacteriol 67: 662–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Vulic M, Keren I, Lewis K. (2012). Role of oxidative stress in persister tolerance. Antimicrob Agents Chemother 56: 4922–4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.