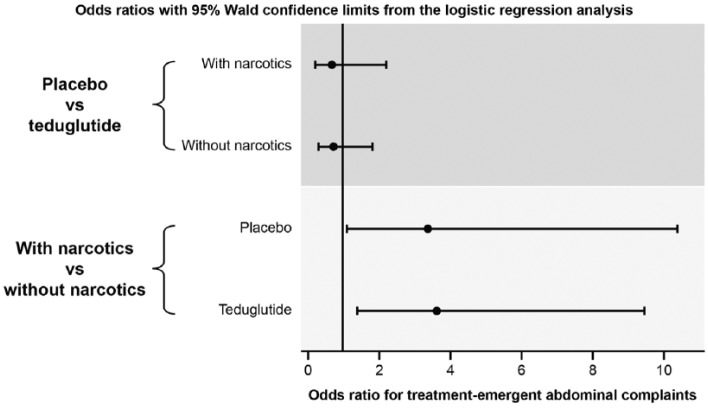
Figure 1.
Correlation of treatment-emergent gastrointestinal complaints with concomitant narcotic use. Pooled data for patients who received ≥1 dose of teduglutide 0.05 mg/kg/d or matching placebo. Upper graph, probability of abdominal adverse events was not significantly different between treatment groups (95% CIs include 1). Lower graph, narcotic use related to a higher adverse event rate (95% CI lower limits >1).