Abstract
The important role of insulin-like growth factor 1 receptor (IGF-1R) in malignant tumors has been well established. Increased IGF-1R activity promotes cancer cell proliferation, migration, and invasion and is associated with tumor metastasis, treatment resistance, poor prognosis, and shortened survival in patients with cancer. However, while IGF-1R has become a promising target for cancer therapy, IGF-1R-targeted therapy is ineffective in unselected patients. It is therefore essential to evaluate IGF-1R expression before treatment in order to identify responsive patients, monitor therapy efficacy, and estimate prognosis. Insulin-like growth factor 1 receptor molecular imaging is an optimal method for assessing the expression of IGF-1R in vivo accurately and noninvasively. In this review, we will summarize the current status of IGF-1R molecular imaging in cancer, in which 5 major classes of ligands that have been developed for noninvasive IGF-1R molecular imaging will be discussed: natural ligands, monoclonal antibodies, antibody fragments, affibodies, and small molecules. For decades, IGF-1R molecular imaging is studied in full swing and more effort is needed in the future.
Keywords: IGF-1R, target, molecular imaging, cancer
Introduction
The insulin-like growth factor (IGF) signaling pathway is a complex network comprising 2 ligands (IGF-1 and IGF-2), 2 receptors (IGF-1R and IGF-2R), and 6 IGF-binding proteins (IGFBP1-6). Insulin-like growth factor 1 receptor is a key player in several physiological processes such as cell growth, proliferation, differentiation, and apoptosis, and it is well documented that IGF-1R plays a critical role in cancer formation, progression, and metastasis.1,2 Preclinical data show that IGF-1R is overexpressed in several malignant tumors including lung cancer,3 breast cancer,4 prostate cancer,5 glioma,6 gastrointestinal cancers,7 and so on. Moreover, clinical research demonstrates that IGF-1R has potent antiapoptotic and transforming activities and that increased IGF-1R activity is associated with tumor metastasis, treatment resistance, poor prognosis, and shortened survival.3,8-10 Insulin-like growth factor 1 receptor has therefore emerged as a potential and promising diagnostic and therapeutic biomarker in cancers.11
To date, over 10 IGF-1R-targeted drugs have been approved for clinical trials. A wide range of clinical effects were reported in these studies, from minor or no clinical benefits to close to complete response. For instance, in a phase I clinical trial on 4 patients with advanced lung squamous cell carcinoma treated for 7 months with IGF-1R-targeting small-molecule inhibitory drugs (picropodophyllin, PPP, AXL1717), none of the patients developed new metastases. Moreover, central necrosis was confirmed in these patients with computed tomography (CT) and 18F-Fludeoxyglucose positron emission tomography (PET).12 However, many studies failed to achieve the desired results. The results of initial phase III studies in unselected patients with anti-IGF-1R monoclonal antibodies were disappointing,13,14 thus highlighting the need to develop effective biomarkers to select responsive patients and predict clinical results more accurately.
Tissue biopsy and immunohistochemistry are currently the most commonly used methods for the detection of IGF-1R; however, they present several limitations. First, tissue biopsy is an invasive method poorly accepted by patients and their families. Second, not all lesions can provide pathological data. Finally, tumor heterogeneity may affect the accuracy of biopsy results.15-17 For instance, different IGF-1R expression levels may be obtained within the same tumor or between the primary tumors and the metastatic lesions. Moreover, IGF-1R expression can change throughout tumor development and also during treatment. There is therefore an urgent need to develop an accurate noninvasive method to detect in vivo IGF-1R expression, in order to screen patients potentially responsive to IGF-IR-targeted treatment, monitor changes in IGF-1R expression levels during treatment, and guide the selection of adequate clinical treatments. In recent years, the development of molecular imaging has allowed the in vivo visualization of cells, molecules, and metabolic processes in real time. Thus, in vivo IGF-1R-targeted imaging could be a valuable tool for determining IGF-1R expression noninvasively.
Insulin-Like Growth Factor 1 Receptor and Cancer
Expression of IGF-1R in tumors
A large number of studies have shown that IGF-1R is upregulated in most malignant tumors and that it plays a crucial role in phenotypic transformation and maintenance. Insulin-like growth factor 1 receptor binding to its natural ligands IGF-1 or IGF-2 activates the PI3K-Akt and Ras-Raf-ERK/MAPK signaling pathways, thereby promoting proliferation, differentiation, migration, and apoptosis inhibition.1,18-20 Insulin-like growth factor 1 receptor activation is closely associated with tumor angiogenesis, metastasis, and treatment resistance.11,21,22 The mechanism may be related to the following aspects: it can modulate cell mitosis, it is required for tumorigenesis, and it could protect tumor cells from apoptosis.23
Insulin-like growth factor 1 receptor and oncogenes
Besides having a direct effect on cellular proliferation and survival, IGF-1R is also a key mediator in the biochemical and molecular events driving oncogenic transformation.11,20,24 The signaling pathways downstream of IGF-1R have multiple crossing sites with oncogenes such as Ras, c-myc, and c-fos, which can in turn regulate each other, ultimately leading to tumor formation. In addition, these IGF-1R downstream signaling pathways can also interact with the epidermal growth factor receptor (EGFR) and vascular EGFR pathways to regulate cell proliferation and differentiation. Activation of IGF-1R causes upregulation of hypoxia-inducible factor 1α protein synthesis, which induces expression of VEGF, a central mediator of angiogenesis.25 Insulin-like growth factor 1 receptor is also closely related to breast cancer gene 1 (BRCA1). Indeed, wild-type BRCA1 expression caused a significant decrease in IGF-1R promoter activity and endogenous IGF-1R levels in breast cancer cell lines, whereas mutant BRCA1 expression had no effect on IGF-1R levels.26
The studies discussed earlier are just a small selection among the large body of research demonstrating the essential role of IGF-1R in malignant transformation. Thus, IGF-1R-targeted imaging has strong potential to become an invaluable tool for tumor diagnostics and therapy efficacy monitoring.
Insulin-Like Growth Factor 1 Receptor–Targeted Molecular Imaging
Insulin-like growth factor 1 and analogue molecular imaging
Insulin-like growth factor 1 was labeled with 125I and its biodistribution was evaluated in tumor-bearing mice.27 Insulin-like growth factor 1 (7649 Da) is the natural ligand of IGF-1R, which makes it have a high affinity to IGF-1R, but IGFBPs in the serum may obstruct IGF-1 from binding to target, which greatly reduced its affinity. When coinjected with cold IGF-1, there appeared to be more 125 I-labeled IGF-1 accumulated in the tumor area and less emerged in normal tissues, maybe due to partial saturation of IGFBPs. However, catabolism occurred rapidly making it not fit for imaging IGF-1R in vivo. The same author28 assessed the feasibility of 125I-labeled des(1-3) IGF-1, a truncated analogue of IGF-1, as a probe for molecular imaging. The probe was quickly internalized after binding to IGF-1R and also rapidly catabolized at 37°C with release of breakdown products either. These preliminary results suggested that 125I-labeled IGF and 125I-labeled des (1-3) IGF-1 had little potential as an imaging probe. Cornelissen et al29 labeled IGF-1 and its analogue IGF-1 (E3R) with 111In and showed that IGF-1 (E3R) uptake was strongly correlated with IGF-1R expression. Moreover, human breast cancer cells (MCF-7)/human epidermal growth factor receptor (HER) 2-18 tumors were clearly visible by micro single-photon emission computed tomography (SPECT), thus suggesting that 111In-labeled IGF-1(E3K) is a promising molecular imaging tracer.
Insulin-like growth factor 1 receptor monoclonal antibody molecular imaging
R1507 is a fully humanized antibody most commonly used in IGF-1R molecular imaging. R1507 labeled with 111In and 125I was assessed with SPECT imaging in nude mice bearing breast cancer SUM149.30 In this study, the tumor was clearly visible with 111In-R1507, and the tumor to background ratio increased gradually with time. Blocking assays with excess cold R1507 showed that 111In-R1507 has high specificity to IGF-1R. Moreover, this probe showed higher tumor uptake than 125I-R1507 (20% ± 6% ID/g vs 8% ± 1% ID/g, 33% ± 6% ID/g vs 7% ± 1% ID/g, 31% ± 4% ID/g vs 5% ± 1% ID/g in 1, 3, and 7 days after injection, respectively).
Positron emission tomography imaging has higher sensitivity and provides more accurate quantitative quantifications than SPECT. A new PET imaging tracer, 89Zr-N-Succinyldesferal-human anti-IGF-1R monoclonal antibody R1507 (89Zr-R1507), was synthesized successfully and imaging in nude mice bearing breast cancer SUM149 was performed. When compared to 111In-R1507 SPECT imaging, the tumor uptake and tumor to blood ratio obtained with 89Zr-R1507 PET imaging were similar (22% ± 3% ID/g vs 20.9% ± 2.4% ID/g and 3.9% ± 0.5% ID/g vs 3.8% ± 0.7% ID/g), but the tumor to liver ratio was slightly lower (4.8% ± 0.2% ID/g vs 7.5% ± 1.2% ID/g).30,31 These studies suggest that IGF-1R monoclonal antibodies labeled with radionuclides could be valuable probes for IGF-1R molecular imaging.
Fluorescence imaging is among the most widely utilized molecular imaging methods. The IGF-1R monoclonal antibody AVE-1642, labeled with either small molecular fluorophores or quantum dot (ODs), was assessed in preclinical models of human breast cancer MCF-7.32 Most ODs were detected in normal organs including the liver, spleen, lymph nodes, and bone marrow, rather than on MCF-7 breast tumors expressing IGF-1R. Moreover, ex vivo data showed that this OD accumulation in the tumor regions was unspecific. In contrast, AVE-1642-conjugated Alexa 680 accumulated in tumor areas without obvious nonspecific uptake, suggesting that this probe may be suitable for molecular imaging. Similar results were obtained in a study examining anti-IGF-1R antibodies (clone 24-31) conjugated with 550- to 650-nm fluorophores in molecular imaging of different subcutaneous and orthotopic pancreatic tumors.33 By further analyzing this fluorescently conjugated IGF-1R antibody in models of subcutaneous colon cancer, in situ colon cancer, and colon cancer liver metastases, the same authors demonstrated that this tracer can identify not only in situ tumor regions but also metastases.34 Together, these studies showed that in situ and metastases tumors may be imaged noninvasively with IGF-1R antibodies conjugated with fluorophores. Fluorescence imaging technology has many advantages such as nonradiation, convenient, and real time, which can accurately reflect changes in disease.35 Although fluorescence imaging had limited depth that restricted its application to deep tumors, fluorescence image-guided surgery is in full swing.36,37 A mount of new fluorescence tracers had successfully been tested in animal studies.38-40 We could expect these preclinical results translating to clinical patients.
In addition, it was demonstrated that monoclonal antibody probes could not only provide a clear imaging of IGF-1R expression in tumors but could also detect IGF-1R expression levels based on probe uptake quantifications. For instance, biodistribution experiments in preclinical models of bone sarcoma tumors with different IGF-1R expression levels (OS-1, EW-5, EW-8, and OS-33) revealed that 111In-R1507 had high radioactivity aggregation in OS-1 (IGF-1R overexpression) and EW-5 (IGF-1R moderate expression) tumors (27.5% ± 6.5% ID/g and 14.0% ± 2.8% ID/g, respectively), whereas IGF-1R low-expression tumors (EW-8 and OS-33) showed low radioactive accumulation (6.5% ± 1.5% ID/g and 5.5% ± 0.6% ID/g, respectively).41 Tumor uptake in these models varied relative to the IGF-1R expression level, with highest uptake in OS-1 and tumor to background ratio gradually increasing with time. As expected, the tumor to blood ratios of OS-1 and EW5 were markedly different. Moreover, probe uptake in different tumors within the same subject was examined in a OS-1/OS-33 tumor-bearing nude mice model. Notably, SPECT imaging revealed that although the OS-1 tumor showed significant radioactive agglomeration, the OS-33 tumor had low tracer uptake. That IGF-1R expression levels could be detected based on probe uptake quantifications was also corroborated by England et al.42 In this study, PET imaging with a new IGF-1R tracer (1A2G11) labeled with 89Zr was performed on pancreatic tumor models with different IGF-1R expression levels: MIA PaCa-2 (overexpression), BxPC-3 1R (moderate expression), and AsPC-1 (low expression). Maximal uptake of 89Zr-Df-1A2G11 measured 2 hours after injection was detected in MIA PaCa-2 tumors (7.28% ± 1.36% ID/g), followed by BxPC-3 (5.27% ± 0.17% ID/g) and AsPC-1 tumors (2.77% ± 0.12% ID/g). MIA PaCa-2 tumors also showed the highest tracer uptake at 24, 48, 72, and 120 hours (Figure 1). This new tracer is therefore a promising candidate for clinical studies aiming to screen patients who may benefit from IGF-1R therapy.
Figure 1.
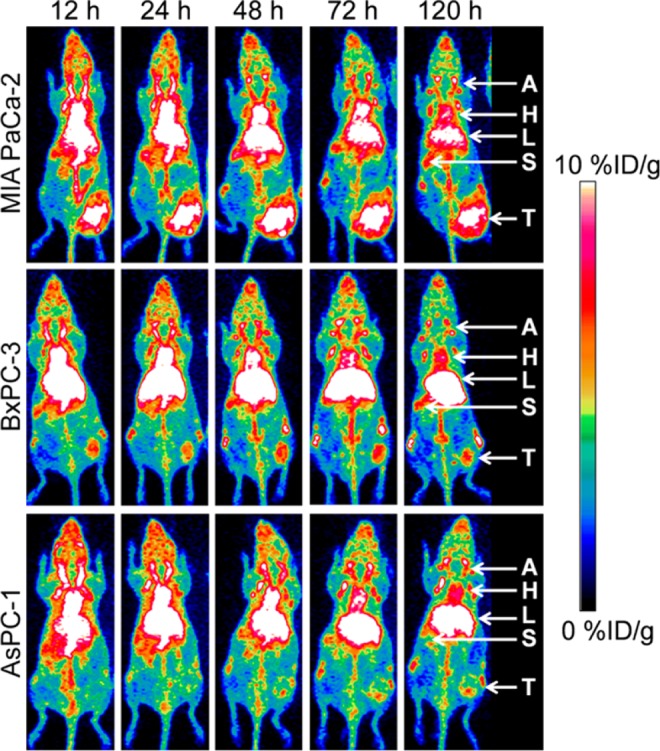
89Zr-Df-1A2G11 positron emission tomography (PET)/computed tomography (CT) imaging was performed in different pancreatic cancer models. MIA PaCa-2 (high insulin-like growth factor 1 receptor [IGF-1R] expression) tumors showed the highest accumulation followed by BxPC-3 (moderate IGF-1R expression). The lowest tumor accumulation was found in AsPC-1 (low IGF-1R expression) tumors. T indicates tumor; A, carotid artery; H, Heart; L, liver; S, spleen. Adapted and reproduced with permission from England et al.42
Insulin-like growth factor 1 receptor antibody fragment molecular imaging
Despite showing some encouraging results in preclinical studies, monoclonal antibodies present many limitations for molecular imaging. As they have a large molecular volume (150 kDa) and hence slow blood clearance rates, monoclonal antibodies produce high imaging background, low tumor to background ratio, and poor imaging quality, in particular, during the first hours following injection. In addition, the large size of monoclonal antibodies also limits their penetration into the tumor area, which reduces probe access to the molecular target, thereby affecting imaging quality. To overcome these technical challenges, a number of researchers have attempted to use antibody fragments instead of whole antibodies. For instance, 111In-F(ab′)2-R1507, an IGF-1R antibody fragment probe developed by Fleuren et al,43 showed high tumor uptake in nude mice model of EW5 tumors by SPECT/CT imaging performed at 2, 4, 8, and 24 hours after injection of the imaging agent. Notably, the tumor to blood ratio at 24 hours was significantly higher than that obtained with 111In-R1507. In addition, 111In-F(ab′)2-R1507 showed significant uptake in EW8 tumors, in contrast to 111In-R1507. This important result demonstrates that smaller antibody fragments are superior to whole molecular imaging probes. Another study compared the imaging quality obtained with different antibody fragment probes: 111In- R1507 IgG, 111In- R1507F(ab′)2, and 111In-R1507 Fab.44 Interestingly, while 111In-R1507 IgG showed the highest tumor uptake in biodistribution assays, the maximal tumor to blood ratio of 111In-R1507F(ab′)2 was over twice as high as that of 111In-R1507 IgG. 111In-R1507 Fab had the lowest tumor uptake and tumor–blood ratio, and the major fraction of the tumor targeting was nonspecific. Moreover, 111In-R1507F(ab′)2 produced the best SPECT imaging quality in nude mice bearing breast cancer SUM149 when compared to the other probes, in particular at 6 hours after injection of the tracer. 111In-R1507F(ab′)2 accumulated in the tumor area rapidly and was then quickly excreted. The smaller antibody fragment (111In-R1507 Fab) had very low affinity to IGF-1R and hence showed little or no uptake in vivo.
Insulin-like growth factor 1 receptor affibody molecular imaging
Affibody molecules are nonimmunoglobulin-based scaffold proteins of small size (7 kDa)45 that with high affinity and specificity make them have great potential as molecular imaging probes.46 Tolmachev et al47 first demonstrated the feasibility of using affibody molecules to evaluate IGF-1R expression in vivo by performing DU145 SPECT imaging in prostate cancer models with the newly developed 111In-DOTA-ZIGF1R: 4551 affibody molecule (Figure 2). Tumor imaging with the probe was clear. However, radioactive accumulation was also detected in normal organs expressing IGF-IR such as salivary glands, lungs, and stomach.
Figure 2.
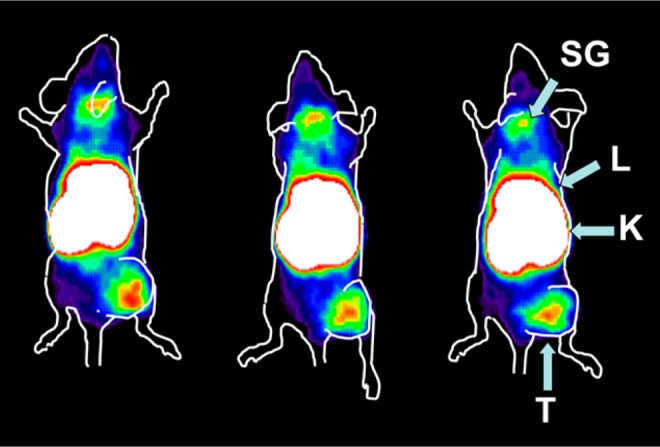
r-Camera imaging was performed by insulin-like growth factor 1 receptor (IGF-1R) expression DU-145 prostate cancer xenografts 8 hours after 111In-DOTA-ZIGF1R:4551 injection. T indicates tumor; K, kidney; L, liver; SG, salivary gland. Adapted and reproduced with permission from Tolmachev et al.47
As 111In has a longer half-life and higher energy than 99mTc, it is expected that SPECT imaging with 99mTc instead of 111In will improve imaging sensitivity and resolution, besides reducing the patient radiation dose. However, SPECT imaging with [99mTc (CO) 3]+-(HE)3-ZIGF1R: 4551 in prostate cancer DU-145 nude mice showed that this tracer still accumulated in the stomach, lung, and salivary glands.48 Nevertheless, significant tumor uptake was detected at 8 hours after probe injection, and the radioactivity accumulation in the liver and spleen was reduced 3.6-fold when compared to 111In-DOTA-ZIGF1R: 4551. This study demonstrated that labeling ZIGF1R: 4551 with 99mTc greatly reduces the probe’s liposoluble and background noise, thus improving imaging quality.
Mitran et al49 designed a molecular imaging probe by modifying the affinity of the ZIGF1R: 4551 affibody molecule. In vitro assays showed that the new probe, ZIGF1R: 4551-GGGC, displayed high specificity and affinity to prostate cancer (DU145) and breast cancer (MCF-7) cell lines which overexpress IGF-1R. Notably, the specificity and affinity of ZIGF1R: 4551-GGGC to IGF-1R was similar to those of its natural ligand, IGF-1. Furthermore, 99mTc-ZIGF1R:4551-GGGC showed excellent tumor to blood ratio in DU145 and MCF-7 tumor models at 4 hours following probe injection. When compared to [99mTc (CO)3]+-(HE)3-ZIGF1R: 4551, 99mTc-ZIGF1R: 4551-GGGC uptake in the kidneys was decreased nearly 16-fold, but liver uptake was increased by 1.2- to 2-fold, demonstrating that 99mTc-ZIGF1R: 4551-GGGC had higher liposoluble than [99mTc (CO)3]+-(HE)3-ZIGF1R: 4551. Both these probes present advantages and limitations. Besides producing low radioactivity accumulation in the liver, the HEHEHE tag of [99mTc (CO)3]+-(HE)3-ZIGF1R: 4551 can conveniently be used for IMAC purification, thus simplifying the production of the probe. On the other hand, the chelating properties of the GGGC motif causes low radioactivity accumulation in the kidneys, although this tag requires more complicated purification schemes during tracer production.50 Follow-up studies should focus on how to reduce the fat solubility of these probes.
In 2010, Li et al51 screened for new IGF-1R-binding affibody molecules with phage-display technology. A promising candidate, Z4:40 (the structure is shown in Figure 3), was shown to specifically and efficiently recognize IGF-1R by different methods including flow cytometry and fluorescence microscopy. In another study, this affibody was labeled with 64Cu for PET/CT imaging (64Cu-NOTA-ZIGF-1R: 4:40) and then assessed in a glioma tumor model (U87MG).52 The tumor was clearly visible 1 to 24 hours after injection of the probe, and the radioactivity uptake was 5.08% ± 1.07% ID/g, with a high tumor to background ratio. Moreover, blocking assays with excess of unlabeled probe showed that 64Cu-NOTA-ZIGF-1R: 4:40 was highly specific. However, radioactivity uptake in the liver but mostly in the kidneys was high, indicating that the probe had high hydrophilicity and was excreted mostly via the urinary system.
Figure 3.

The structure of affibody ZIGF-1R:4:40 and small-molecule GSK1838705A.
Small molecule-based thymidine kinase receptor inhibitor molecular imaging
Small molecule-based probes could achieve high affinity, selectivity, and adequate lipophilicity, which make them the most potential and vintage molecular tracers. To our knowledge, 18F-BMS-754807 was the first potential small-molecule tracer for cancer IGF-1R imaging.53,54 It had been proved by autoradiography studies that 18F-BMS-754807 could bind to IGF-1R of surgically removed human glioblastoma grade IV, breast cancer, and pancreatic tumor with a high affinity. Consecutively, imaging of rat and mice was undergone. The heart and pancreas where IGF-1R is proved to be present showed significant tracer accumulation; however, no notable activity was found in brain, which indicates that it may just potentially be a tumor radiotracer outside the brain. Another molecule tracer, [11C]GSK1838705A55 (the structure is shown in Figure 3) was synthesized successfully and showed a higher affinity to U87MG cells in vitro. Micro-PET imaging in C57BL/6 mice indicated that it could enter the brain through blood–brain barrier (BBB), which make it possible to image the intracranial tumors overexpressed IGF-1R in vivo.
Perspectives in IGF-1R Molecular Imaging
As IGF-1R is overexpressed in a variety of tumors, IGF-1R expression could be used to screen patients who may benefit from IGF-1R-targeted therapy as well as to monitor therapeutic efficacy and estimate prognosis. In recent years, there have been a growing number of studies devoted to IGF-1R-targeting molecular imaging research. The author believes that the future directions for the development of IGF-1R-targeted molecular imaging include the following aspects.
Molecular probe
A molecular probe with high stability, high affinity, and high specificity is an important prerequisite for molecular imaging, and the choice of probe determines the imaging feasibility and imaging quality. Because of their large molecular size, antibody and antibody fragment probes display slow tumor accumulation and blood circulation clearance. Affibodies may be superior to antibody due to their relative small size, stable chemical properties, and high affinity to the target. But according to the experience of EGFR-targeted molecular imaging research, small-molecule probes may be the most promising one because of its small size and high affinity and specificity.
A variety of potential IGF-1R ligands cannot pass through the BBB, which limits their clinical application in brain tumors. It is therefore necessary to develop new IGF-1R-binding probes that can cross the BBB and provide high-quality molecular imaging. Moreover, a lot of probes have high lipophilicity and are excreted via the liver and gallbladder, which make them not the perfect choice to imaging abdominal tumors because of the high background. Thus, hydrophilicity needs to be improved while decreasing lipophilicity in order to reduce nonspecific uptake in organs of the abdomen and improve the imaging quality of abdominal tumors.
Imaging equipment
Single-photon emission computed tomography is more widely used and costs less than PET.56 And, the probe of SPECT is more easy to synthesize. However, PET has higher sensitivity, thus providing more accurate quantitative analyses. When combined with specific and efficient tracers, PET can be considered an optimal quantitative metabolic imaging technique. More research is needed for developing new PET targeting molecular imaging probes.
Tumor models
While the various studies discussed in this review mostly used subcutaneous tumor models to assess molecular imaging probes, the microenvironments of orthotopic and subcutaneous tumors differ considerably. Future molecular imaging studies should focus on orthotopic tumor models in order to provide a solid theoretical basis for clinical translation.
Conclusion
Molecular targeted therapy is the future of cancer treatment. Clinical research on IGF-1R targeting therapy shows that modulating IGF-1R expression has significant therapeutic effects. Insulin-like growth factor 1 receptor molecular imaging may provide more accurate information in quantification of IGF-1R expression in vivo, which would provide a reliable basis for screening patients for targeted therapy as well as for evaluating the effects of treatment and estimating prognosis. The development of more high-affinity and high-specific probes would open the way for the clinical translation of IGF-1R molecular imaging. In a future scenario, the replacement of imaging radionuclides with therapeutic radionuclides would allow simultaneous tumor imaging and local radiation therapy. Such a therapeutic strategy could be a breakthrough in cancer treatment, and its added clinical benefits would significantly improve the lives of patients and their families.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Research was supported by the National Basic Research Program of China (2015CB931800), National Natural Science Foundation of China (81471724, 31210103913), and the Key Laboratory of Molecular Imaging Foundation (College of Heilongjiang Province).
References
- 1. Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4(7):505–518. [DOI] [PubMed] [Google Scholar]
- 2. Werner H. For debate: the pathophysiological significance of IGF-I receptor overexpression: new insights. Pediatr Endocrinol Rev. 2009;7(1):2–5. [PubMed] [Google Scholar]
- 3. Yeo CD, Park KH, Park CK, et al. Expression of insulin-like growth factor 1 receptor (IGF-1R) predicts poor responses to epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors in non-small cell lung cancer patients harboring activating EGFR mutations. Lung cancer. 2015;87(3):311–317. [DOI] [PubMed] [Google Scholar]
- 4. Sun WY, Yun HY, Song YJ, et al. Insulin-like growth factor 1 receptor expression in breast cancer tissue and mammographic density. Mol Clin Oncol. 2015;3(3):572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pollak M, Beamer W, Zhang JC. Insulin-like growth factors and prostate cancer. Cancer Metastasis Rev. 1998;17(4):383–390. [DOI] [PubMed] [Google Scholar]
- 6. Trojan J, Cloix JF, Ardourel MY, Chatel M, Anthony DD. Insulin-like growth factor type I biology and targeting in malignant gliomas. Neuroscience 2007;145(3):795–811. [DOI] [PubMed] [Google Scholar]
- 7. Nakajima N, Kozu K, Kobayashi S, et al. The expression of IGF-1R in Helicobacter pylori-infected intestinal metaplasia and gastric cancer. J Clin Biochem Nutr. 2016;59(1):53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heskamp S, Boerman OC, Molkenboer-Kuenen JD, et al. Upregulation of IGF-1R expression during neoadjuvant therapy predicts poor outcome in breast cancer patients. PloS One. 2015;10(2):e0117745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Iams WT, Lovly CM. Molecular pathways: clinical applications and future direction of insulin-like growth factor-1 receptor pathway blockade. Clin Cancer Res. 2015;21(9):4270–4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Riedemann J, Macaulay VM. IGF1R signalling and its inhibition. Endocr Relat Cancer 2006;13(suppl 1):S33–S43. [DOI] [PubMed] [Google Scholar]
- 11. Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8(12):915–928. [DOI] [PubMed] [Google Scholar]
- 12. Ekman S, Frodin JE, Harmenberg J, et al. Clinical phase I study with an insulin-like growth factor-1 receptor inhibitor: experiences in patients with squamous non-small cell lung carcinoma. Acta Oncol. 2011;50(3):441–447. [DOI] [PubMed] [Google Scholar]
- 13. Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer. 2012;12(3):159–169. [DOI] [PubMed] [Google Scholar]
- 14. Fagan DH, Uselman RR, Sachdev D, Yee D. Acquired resistance to tamoxifen is associated with loss of the type I insulin-like growth factor receptor: implications for breast cancer treatment. Cancer Res. 2012;72(13):3372–3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bambury RM, Power DG, O’Reilly S. Intratumor heterogeneity and branched evolution. N Engl J Med. 2012;366:2132; author reply 2133. [DOI] [PubMed] [Google Scholar]
- 16. Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366(10):883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Swanton C. Intratumor heterogeneity: evolution through space and time. Cancer Res. 2012;72(19):4875–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Frasca F, Pandini G, Sciacca L, et al. The role of insulin receptors and IGF-I receptors in cancer and other diseases. Arch Physiol Biochem. 2008;114(1):23–37. [DOI] [PubMed] [Google Scholar]
- 19. Tao Y, Pinzi V, Bourhis J, Deutsch E. Mechanisms of disease: signaling of the insulin-like growth factor 1 receptor pathway—therapeutic perspectives in cancer. Nat Clin Pract Oncol. 2007;4(10):591–602. [DOI] [PubMed] [Google Scholar]
- 20. Werner H, Bruchim I. The insulin-like growth factor-I receptor as an oncogene. Arch Physiol Biochem. 2009;115(2):58–71. [DOI] [PubMed] [Google Scholar]
- 21. Chen HX, Sharon E. IGF-1R as an anti-cancer target—trials and tribulations. Chin J Cancer. 2013;32(5):242–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Denduluri SK, Idowu O, Wang Z, et al. Insulin-like growth factor (IGF) signaling in tumorigenesis and the development of cancer drug resistance. Genes Dis. 2015;2(1):13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Resnicoff M, Burgaud JL, Rotman HL, Abraham D, Baserga R. Correlation between apoptosis, tumorigenesis, and levels of insulin-like growth factor I receptors. Cancer Res. 1995;55(17):3739–3741. [PubMed] [Google Scholar]
- 24. Lann D, LeRoith D. The role of endocrine insulin-like growth factor-I and insulin in breast cancer. J Mammary Gland Biol Neoplasia. 2008;13(4):371–379. [DOI] [PubMed] [Google Scholar]
- 25. Fukuda R, Hirota K, Fan F, Jung YD, Ellis LM, Semenza GL. Insulin-like growth factor 1 induces hypoxia-inducible factor 1-mediated vascular endothelial growth factor expression, which is dependent on MAP kinase and phosphatidylinositol 3-kinase signaling in colon cancer cells. J Biol Chem. 2002;277(41):38205–38211. [DOI] [PubMed] [Google Scholar]
- 26. Abramovitch S, Glaser T, Ouchi T, Werner H. BRCA1-Sp1 interactions in transcriptional regulation of the IGF-IR gene. FEBS letters. 2003;541(1-3):149–154. [DOI] [PubMed] [Google Scholar]
- 27. Sun BF, Kobayashi H, Le N, et al. Effects of insulinlike growth factor binding proteins on insulinlike growth factor-I biodistribution in tumor-bearing nude mice. J Nucl Med. 2000;41(2):318–326. [PubMed] [Google Scholar]
- 28. Sun BF, Kobayashi H, Le N, et al. Biodistribution of 125I-labeled des(1-3) insulin-like growth factor I in tumor-bearing nude mice and its in vitro catabolism. Cancer Res. 1997;57(13):2754–2759. [PubMed] [Google Scholar]
- 29. Cornelissen B, McLarty K, Kersemans V, Reilly RM. The level of insulin growth factor-1 receptor expression is directly correlated with the tumor uptake of (111)In-IGF-1(E3 R) in vivo and the clonogenic survival of breast cancer cells exposed in vitro to trastuzumab (Herceptin). Nucl Med Bio. 2008;35(6):645–653. [DOI] [PubMed] [Google Scholar]
- 30. Heskamp S, van Laarhoven HW, Molkenboer-Kuenen JD, et al. ImmunoSPECT and immunoPET of IGF-1R expression with the radiolabeled antibody R1507 in a triple-negative breast cancer model. J Nucl Med. 2010;51(10):1565–1572. [DOI] [PubMed] [Google Scholar]
- 31. Leung K. 89Zr-N-Succinyldesferal-human anti-insulin-like growth factor 1 receptor monoclonal antibody R1507. Molecular Imaging and Contrast Agent Database (MICAD), Bethesda, MD: National Center for Biotechnology Information (US); 2004. [PubMed] [Google Scholar]
- 32. Zhang H, Zeng X, Li Q, Gaillard-Kelly M, Wagner CR, Yee D. Fluorescent tumour imaging of type I IGF receptor in vivo: comparison of antibody-conjugated quantum dots and small-molecule fluorophore. Br J Cancer. 2009;101(1):71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Park JY, Lee JY, Zhang Y, Hoffman RM, Bouvet M. Targeting the insulin growth factor-1 receptor with fluorescent antibodies enables high resolution imaging of human pancreatic cancer in orthotopic mouse models. Oncotarget. 2016;7(14):18262–18268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Park JY, Murakami T, Lee JY, Zhang Y, Hoffman RM, Bouvet M. Fluorescent-antibody targeting of insulin-like growth factor-1 receptor visualizes metastatic human colon cancer in orthotopic mouse models. PloS One. 2016;11(1):e0146504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ballou B, Ernst LA, Waggoner AS. Fluorescence imaging of tumors in vivo. Curr Med Chem. 2005;12(7):795–805. [DOI] [PubMed] [Google Scholar]
- 36. Digonnet A, van Kerckhove S, Moreau M, et al. Near infrared fluorescent imaging after intravenous injection of indocyanine green during neck dissection in patients with head and neck cancer: A feasibility study. Head Neck. 2016;38(suppl 1):E1833–E1837. [DOI] [PubMed] [Google Scholar]
- 37. Schaafsma BE, Mieog JS, Hutteman M, et al. The clinical use of indocyanine green as a near-infrared fluorescent contrast agent for image-guided oncologic surgery. J Surg Oncol. 2011;104(3):323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mieog JS, Vahrmeijer AL, Hutteman M, et al. Novel intraoperative near-infrared fluorescence camera system for optical image-guided cancer surgery. Mol Imaging. 2010;9(4):223–231. [PubMed] [Google Scholar]
- 39. Adams KE, Ke S, Kwon S, et al. Comparison of visible and near-infrared wavelength-excitable fluorescent dyes for molecular imaging of cancer. J Biomed Opt. 2007;12(2):024017. [DOI] [PubMed] [Google Scholar]
- 40. Kuil J, Velders AH, van Leeuwen FW. Multimodal tumor-targeting peptides functionalized with both a radio- and a fluorescent label. Bioconjug Chem. 2010;21(10):1709–1719. [DOI] [PubMed] [Google Scholar]
- 41. Fleuren ED, Versleijen-Jonkers YM, van de Luijtgaarden AC, et al. Predicting IGF-1R therapy response in bone sarcomas: immuno-SPECT imaging with radiolabeled R1507. Clin Cancer Res. 2011;17(24):7693–7703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. England CG, Kamkaew A, Im HJ, et al. ImmunoPET imaging of insulin-like growth factor 1 receptor in a subcutaneous mouse model of pancreatic cancer. Mol Pharm. 2016;13(6):1958–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fleuren ED, Versleijen-Jonkers YM, Heskamp S, et al. The strength of small: improved targeting of insulin-like growth factor-1 receptor (IGF-1R) with F(ab’)(2)-R1507 fragments in Ewing sarcomas. Eur J Cancer. 2013;49(13):2851–2858. [DOI] [PubMed] [Google Scholar]
- 44. Heskamp S, van Laarhoven HW, Molkenboer-Kuenen JD, et al. Optimization of IGF-1R SPECT/CT imaging using 111In-labeled F(ab’)2 and Fab fragments of the monoclonal antibody R1507. Mol Pharm. 2012;9(8):2314–2321. [DOI] [PubMed] [Google Scholar]
- 45. Ahlgren S, Orlova A, Rosik D, et al. Evaluation of maleimide derivative of DOTA for site-specific labeling of recombinant affibody molecules. Bioconjug Chem. 2008;19(1):235–243. [DOI] [PubMed] [Google Scholar]
- 46. Lofblom J, Feldwisch J, Tolmachev V, Carlsson J, Stahl S, Frejd FY. Affibody molecules: engineered proteins for therapeutic, diagnostic and biotechnological applications. FEBS lett. 2010;584(12):2670–2680. [DOI] [PubMed] [Google Scholar]
- 47. Tolmachev V, Malmberg J, Hofstrom C, et al. Imaging of insulinlike growth factor type 1 receptor in prostate cancer xenografts using the affibody molecule 111In-DOTA-ZIGF1R:4551. J Nucl Med. 2012;53(1):90–97. [DOI] [PubMed] [Google Scholar]
- 48. Orlova A, Hofstrom C, Strand J, et al. [99mTc(CO)3]+-(HE)3-ZIGF1R:4551;a new Affibody conjugate for visualization of insulin-like growth factor-1 receptor expression in malignant tumours. Eur J Nucl Med Mol Imaging. 2013;40(3):439–449. [DOI] [PubMed] [Google Scholar]
- 49. Mitran B, Altai M, Hofstrom C, et al. Evaluation of 99mTc-Z IGF1R:4551-GGGC affibody molecule, a new probe for imaging of insulin-like growth factor type 1 receptor expression. Amino Acids. 2015;47(2):303–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wallberg H, Lofdahl PK, Tschapalda K, et al. Affinity recovery of eight HER2-binding affibody variants using an anti-idiotypic affibody molecule as capture ligand. Protein Expr Purif. 2011;76(1):127–135. [DOI] [PubMed] [Google Scholar]
- 51. Li J, Lundberg E, Vernet E, Larsson B, Hoiden-Guthenberg I, Graslund T. Selection of affibody molecules to the ligand-binding site of the insulin-like growth factor-1 receptor. Biotechnol Appl Biochem. 2010;55(2):99–109. [DOI] [PubMed] [Google Scholar]
- 52. Su X, Cheng K, Liu Y, Hu X, Meng S, Cheng Z. PET imaging of insulin-like growth factor type 1 receptor expression with a 64Cu-labeled affibody molecule. Amino Acids. 2015;47(7):1409–1419. [DOI] [PubMed] [Google Scholar]
- 53. Majo VJ, Arango V, Simpson NR, et al. Synthesis and in vitro evaluation of [18F]BMS-754807: a potential PET ligand for IGF-1R. Bioorg Med Chem Lett. 2013;23(14):4191–4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Prabhakaran J, Dewey SL, McClure R, et al. In vivo evaluation of IGF1R/IR PET ligand [18F]BMS-754807 in rodents. Bioorg Med Chem Lett. 2017;27(4):941–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Solingapuram Sai KK, Prabhakaran J, Sattiraju A, Mann JJ, Mintz A, Kumar JSD. Radiosynthesis and evaluation of IGF1R PET ligand [11C]GSK1838705A. Bioorg Med Chem Lett. 2017;27(13):2895–2897. [DOI] [PubMed] [Google Scholar]
- 56. Simanek M, Koranda P. SPECT/CT imaging in breast cancer—current status and challenges. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2016;160(4):474–483. [DOI] [PubMed] [Google Scholar]


