Abstract
A total of two postpartum women with no noteworthy medical history presented with persistent headache. Brain magnetic resonance imaging (MRI) of both revealed extensive cerebral venous thrombosis, concurrently with abnormal signals of the splenium of the corpus callosum (SCC): The splenium appeared hyperintense on T2-weighted sequences, fluid-attenuated inversion recovery (FLAIR), and diffusion-weighted imaging (DWI) imaging, and hypointense on T1 sequences and apparent diffusion coefficient (ADC) map. The patients were given thrombolytic therapy. Clinically, both patients achieved recovery with no neurologic sequelae, and follow-up MRI revealed complete resolution of the lesion in the SCC at day 36 and day 37 after initial presentation, respectively.
Keywords: cerebral venous thrombosis, MRI, RESLES, splenium corpus callosum
Introduction
Reversible splenial lesion syndrome (RESLES) is a disorder characterized by the presence of a focal lesion often involving the central area of the splenium of the corpus callosum (SCC), followed by complete reversibility on follow-up magnetic resonance imaging (MRI) after a variable period of time. It is more commonly seen among patients with encephalitis or encephalopathy and among patients receiving antiepileptic drugs.1
We report two cases of postpartum patients with cerebral venous thrombosis (CVT) concurrently presenting with an oval lesion of the SCC on MRI, hyperintense on T2-weighted and fluid-attenuated inversion recovery (FLAIR) sequences. The lesions appeared on the 10th and 4th day of initial onset, respectively, and remitted on the follow up 1 month later. Therefore, a diagnosis of RESLES was made. Additionally, we discuss the potential pathophysiological mechanism of RESLES, with respect to the results of previous studies.
Treatment procedures for both patients were standard and in accordance with the Helsinki Declaration as revised in 2013. Both patients have given written consent for publication of their cases and imaging data in an international medical journal.
Case presentation
Patient 1
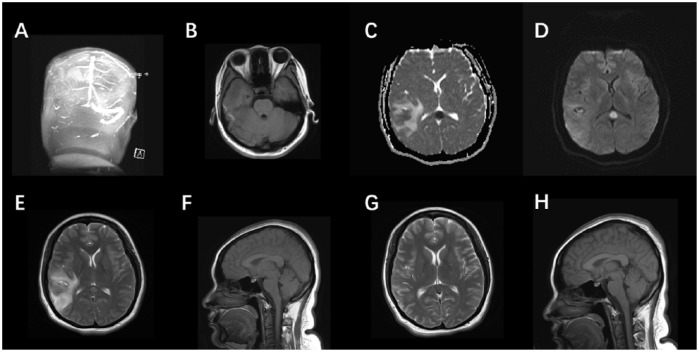
A 29-year-old woman presented with persistent right temporal parietal headache at 42 days postpartum. She had no noteworthy medical history and was not taking any medications. Neurological evaluation was unremarkable. Fundoscopy showed right papilledema. Blood electrolytes were unremarkable at onset. Computed tomography scan indicated “right temporal lobe hemorrhage”. She was admitted to local hospital. Initial MRI of the brain revealed a heterogeneous mixed signal of the right temporal lobe and abnormal enhancement of the right tentorial edge nodules. Treatment included lowering intracranial pressure, improving brain cell metabolism, and pain relief. During treatment, she had a temperature up to 99.5°F. Over several days, she experienced improvement of symptoms and a follow-up MRI (day 10 after onset) revealed “right temporal lobe hemorrhage surrounded by apparent swelling, right ventricle deformation due to compression, midline shift to the left, abnormal signal of the right transverse sinus and sigmoid sinus, hypointense on T1 sequence, hyperintense on T2 sequence, suspected cerebral venous thrombus. Abnormal signal of the SCC, right tentorial edge nodules”. Magnetic resonance venography scan showed no flow in the right transverse sinus and sigmoid sinus, suggesting intracranial venous sinus thrombosis. Brain MRI of the lesion in the SCC showed a round lesion, hyperintense on T2-weighted, FLAIR sequence, and diffusion-weighted imaging (DWI) imaging. It was hypointense on T1 sequence and the apparent diffusion coefficient (ADC) map, suggesting the presence of a cytotoxic edema (Figure 1). She was transferred to Beijing Tiantan Hospital Comprehensive Stroke Center for further treatment. Most laboratory tests were normal except D-dimer, which was elevated (4.6 μg/ml, normal < 1.5 μg/ml). The patient was treated with warfarin, mannitol, and prophylactic antiepileptic drugs. Symptoms soon remitted and she was discharged on day 19 after onset. After 2 weeks (total of 37 days after initial presentation) the patient had no neurologic sequelae, and a follow-up MRI revealed complete resolution of the lesion in the SCC.
Figure 1.
(a–h): There is no flow within the right transverse sinus and sigmoid sinus of the source image of magnetic resonance venography (MRV) (a). On T1-weighted image (b) the thrombus within the right transverse sinus is directly visualized as hyperintense clot. Brain MRI showing an oval lesion in the SCC, hyperintense on DWI (c) and T2-weighted (e), and hypointense on the ADC map (d). Sagittal T1W1 (f) displays the same lesion, with slight hypointense signal. Axial T2W1 (e) shows mixed intensity signal in the right temporal lobe, indicating venous thrombosis with secondary hemorrhagic infarct. In the follow up 2 weeks later, note the regression of hemorrhagic lesion in T2W1 (g). The abnormal signal in corpus callosum also remitted completely (g, h).
ADC, apparent diffusion coefficient; DWI, diffusion-weighted imaging; MRI, magnetic resonance imaging.
Patient 2
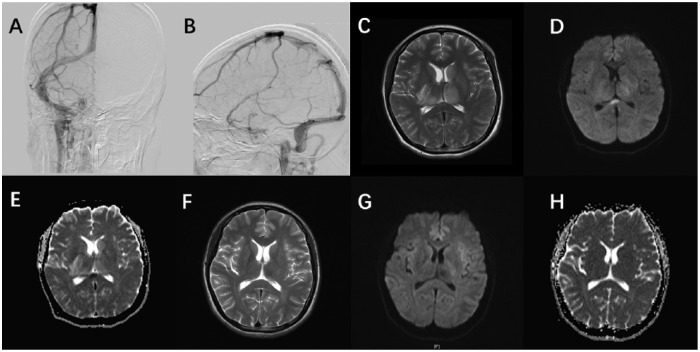
A 27-year-old woman, in prior good health, started with a fever of 100.4°F 2 days postpartum. The pregnancy was without any symptoms or complications. At 7 days postpartum, she experienced headaches at the occipital and parietal area, with increased perspiration and left-sided numbness. Her mental state gradually deteriorated over the next several days. Blood electrolytes were unremarkable at onset. A brain MRI scan 4 days after onset indicated “Weak image signals of the inferior sagittal sinus, the left transverse sinus, sigmoid sinus, straight sinus and the torcular herophili, suspected cerebral venous thrombus. Abnormal signal of the SCC”. Magnetic resonance venography scan showed no flow in the inferior sagittal sinus, the left transverse sinus, sigmoid sinus, straight sinus and the torcular herophili, suggesting intracranial venous sinus thrombosis. The MRI showed a nonenhancing, rounded lesion of the SCC (Figure 2). A diffusion-weighted acquisition showed a hyperintense lesion corresponding to a decreased ADC. A lumbar puncture at day 5 after onset showed 14 leukocytes/μL, glucose level normal, pressure unknown. D-dimer levels were also elevated at 3.3 μg/ml. Her conditions improved in 2 weeks after receiving interventional thrombolytic therapy and further treated with heparin and warfarin. She was discharged with warfarin for further recovery at Beijing Rehabilitation Center. No special treatment was given on account of the lesion in the SCC. A follow-up MRI scan indicated the lesion in the SCC had completely resolved 1.5 weeks after discharge (total of 36 days after initial presentation).
Figure 2.
(a–h): There is no flow within the left transverse sinus, sigmoid sinus (a) and straight sinus (b) of the source image of DSA. An oval lesion is seen hyperintense on axial T2-weighted sequence (c) and DWI (d), hypointense on ADC map (e). The lesion completely remitted in the follow-up MRI (f–h) ten days later.
ADC, apparent diffusion coefficient; DWI, diffusion-weighted imaging; MRI, magnetic resonance imaging.
Discussion
RESLES is a disorder involving transient lesions in the SCC.1 It was first reported by Kim and colleagues2 in 1999, describing a group of epileptic patients with concurrent corpus callosum lesions, deducing that these lesions may be caused by the use of the antiepileptic drug. Since then, there have been other cases of reversible lesions of the corpus callosum reported, but not entirely limited to patients using the antiepileptic drug. Japanese scholars Tada and colleagues3 in 2004 first proposed the clinical symptoms associated with the corpus callosum lesions with reversible mild encephalitis/encephalopathy. Then Garcia-Monco and colleagues4 proposed the concept of reversible corpus callosum lesion syndrome (RESLES) in 2011, and this concept has been in use ever since.
RESLES is most commonly associated with seizures and antiepileptic drugs. Other factors include infections, metabolic conditions, other pharmacological agents, and miscellaneous conditions including anorexia nervosa, malnutrition, vitamin B12 deficiency, Charcot–Marie–Tooth disease, high-altitude cerebral edema, systemic lupus erythematosus (SLE), and eclampsia.5 Clinical presentation is nonspecific, mainly related to the concurrent event, and most frequently presenting as an encephalopathy or encephalitis without evidence of callosal disconnection syndromes. The typical imaging presentation is a nonenhanced rounded or oval lesion in the splenium, hyperintense on T2WI and FLAIR sequence, isointense to slight hypointense on T1WI. DWI showed restricted diffusion with decreased ADC map values.1,6 In certain cases, the lesion shows complete involvement of the splenium on diffusion-weighted image, otherwise known as the ‘boomerang sign’. The pathophysiology of RESLES is obscure. Many RESLES cases shows data supporting that intramyelinic cytotoxic edema plays an important role, as inferred by restricted diffusion on DWI and low ADC values.1,7 Subsequent reversal of ADC values separates these lesions from persistent ischemia. However, it is reported that a neonate with asphyxia showed a reversible lesion on the SCC.8 Considering myelination of the SCC is not completed in the first 2 months of a neonate, intramyelinic edema is unlikely the mechanism in that case. Antiepileptic drug toxicity and association with transhemispheric seizure propagation combined with changes in salt homeostasis and resultant myelin edema are other suggested mechanisms.4,7 There are also some findings in hypoglycemia causing similar reversible lesions on MRI. A common mechanism has not yet been suggested because of the heterogeneous nature of etiologies. Generally, RESLES has an excellent prognosis, resulting in complete recovery without neurological sequelae after the acute disease course.8 The results of previous studies indicate that severe disturbance of consciousness at onset, extracallosal lesions and diffuse slow waves on electroencephalogram (EEG) are indicative of a poor outcome.9 MRI findings are expected to regress spontaneously within a few weeks to months.
These are two of the few reported cases on RESLES in the setting of cerebral venous thrombosis (CVT). CVT is typically seen in young but otherwise previously healthy women. An abundance of predisposing factors of CVT has been identified. The most important ones are a prothrombotic state, commonly seen after routine intake of oral contraceptives, last trimester of pregnancy, puerperium, and ear, nose and throat infections.10 The superior sagittal sinus and the transverse sinus are most commonly involved, frequently seen concurrently in a single patient. The predominant MRI findings for CVT are hyperintense parenchymal lesions on a T2-weighted image that involve white matter, gray matter, or both. Mark E Mullins and colleagues11 did a retrospective study of parenchymal abnormalities associated with CVT, in which they noticed that CVT of the deep veins was associated with deep gray nuclei and deep white matter abnormalities, more commonly involving the thalamus, whereas superficial CVT was associated with cortical and subcortical lesions, often seen in frontal and parietal lobes. Both vasogenic and cytotoxic edema are thought to occur in the setting of CVT.12 Increased venous pressure may lead to increased intracranial pressure, causing breakdown of the blood–brain barrier and vasogenic edema, or may cause severely decreased cerebral blood flow and cytotoxic edema. In the study, seven patients had lesions with elevated ADC values, most likely representing cytotoxic edema, that resolved on follow-up images. They hypothesize that revascularization of thrombosed veins or remodeling of collateral circulation can lead to a decrease in lesion volume, recovering vessel metabolism. Thus, cytotoxic edema associated with CVT may be reversible if the blood drains through collateral pathways.13 In this sense, the reversible lesion in the SCC as seen in our two patients can be explained by the anatomical factors. The corpus callosum has a rich and complicated venous system. Venous drainage of the genu of corpus callosum is by anterior septal veins which are formed by a number of small veins; the two anterior septal veins unite near foramen of Monro and drain into internal cerebral veins, followed by posterior septal veins which also joins into the internal cerebral veins. The alternative drainage of anterior and posterior collosal veins along with numerous anastomotic small vessels give the corpus callosum sufficient circulatory compensation, allowing vessel recovery.
Simone Appenzeller and colleagues reported an active SLE patient with focal transient lesions of the SCC also presenting with CVT on MRI.14 However, the other two patients reported in the study are active SLE patients with SCC reversible lesions without the presence of CVT, indicating that SLE alone may also lead to RESLES, making the true etiology of the patient unclear. Therefore, to our knowledge, we are the first to report cases of CVT with concurrent RESLES. The specific mechanism of RESLES is unknown, however, the reversible pattern on DWI separates these lesions from persistent ischemia and venous sinus occlusion, where ADC reduction followed by reversal is uncommon.1 Considering cytotoxic edema is known to occur in the setting of CVT, both patients had an initial MRI indicating cytotoxic edema in the SCC, and that anatomically the corpus callosum is capable of reversing cytotoxic edema, presenting with a reversible lesion in the MRI. Therefore, we consider cytotoxic edema to be the main mechanism for the two CVT patients to coincide with RESLES. Important etiological causes of splenial lesions in peripartum cases are sinus vein thrombosis, post-ictal state and preeclapmsia-eclampsia.15 Takahashi and colleagues reported a rare case of RESLES with postpartum cerebral angiopathy.16 The predilection for lesions in the SCC is unclear, but it may be related to its relative lack of pressure drop along the vessels, making it susceptible to edema in the setting of hypoxic cerebral vasodilatory circumstances.17 The precise frequency of this syndrome is difficult to ascertain but it is likely underreported, given the time variability of appearance in imaging. Generally, the outcome appears to be completely reversible and necessitated no specific treatment or means of investigation other than a control brain MRI.
In summary, clinicians should be aware that focal reversible lesions in the SCC may be seen in patients with CVT. Patients should be carefully investigated with MRI, including diffusion-weighted images and apparent diffusion coefficient value, and followed with repeated MRI to determine whether the presence of such lesions is transient. Further study of future cases may contribute to our understanding of RESLES.
Footnotes
Funding: This work was supported in part by the National Natural Science Foundation of China (#81501004), and by grant No. Z161100004916104 from the Beijing Municipal Science & Technology Commission.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Jingyi Liu, Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China.
Dacheng Liu, Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China.
Bo Yang, Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China.
Jing Yan, Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China.
Yuehua Pu, Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China.
Jing Zhang, Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China.
Miao Wen, Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China.
Zhonghua Yang, Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China.
Liping Liu, Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, No.6 Tiantan Xili, Dongcheng District, Beijing, 100050, China.
References
- 1. Garcia-Monco JC, Cortina IE, Ferreira E, et al. Reversible splenial lesion syndrome (RESLES): what’s in a name? J Neuroimaging 2011; 21: e1–e14. [DOI] [PubMed] [Google Scholar]
- 2. Kim SS, Chang KH, Kim ST, et al. Focal lesion in the splenium of the corpus callosum in epileptic patients: antiepileptic drug toxicity? AJNR Am J Neuroradiol 1999; 20: 125–129. [PubMed] [Google Scholar]
- 3. Shiihara T, Kato M, Hayasaka K. Clinically mild encephalitis/encephalopathy with a reversible splenial lesion. Neurology 2005; 64: 1487; author reply. [PubMed] [Google Scholar]
- 4. Prilipko O, Delavelle J, Lazeyras F, et al. Reversible cytotoxic edema in the splenium of the corpus callosum related to antiepileptic treatment: report of two cases and literature review. Epilepsia 2005; 46: 1633–1636. [DOI] [PubMed] [Google Scholar]
- 5. Maeda M, Tsukahara H, Terada H, et al. Reversible splenial lesion with restricted diffusion in a wide spectrum of diseases and conditions. J Neuroradiol 2006; 33: 229–236. [DOI] [PubMed] [Google Scholar]
- 6. Gilder TR, Hawley JS, Theeler BJ. Association of reversible splenial lesion syndrome (RESLES) with anti-VGKC autoantibody syndrome: a case report. Neurol Sci 2016; 37: 817–819. [DOI] [PubMed] [Google Scholar]
- 7. Doherty MJ, Jayadev S, Watson NF, et al. Clinical implications of splenium magnetic resonance imaging signal changes. Arch Neurol 2005; 62: 433–437. [DOI] [PubMed] [Google Scholar]
- 8. Takanashi J, Maeda M, Hayashi M. Neonate showing reversible splenial lesion. Arch Neurol 2005; 62: 1481–1482; author reply 2. [DOI] [PubMed] [Google Scholar]
- 9. Zhang S, Ma Y, Feng J. Clinicoradiological spectrum of reversible splenial lesion syndrome (RESLES) in adults: a retrospective study of a rare entity. Medicine (Baltimore) 2015; 94: e512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Linn J, Bruckmann H. Cerebral venous and dural sinus thrombosis*: state-of-the-art imaging. Clin Neuroradiol 2010; 20: 25–37. [DOI] [PubMed] [Google Scholar]
- 11. Mullins ME, Grant PE, Wang B, et al. Parenchymal abnormalities associated with cerebral venous sinus thrombosis: assessment with diffusion-weighted MR imaging. AJNR Am J Neuroradiol 2004; 25: 1666–1675. [PMC free article] [PubMed] [Google Scholar]
- 12. Forbes KP, Pipe JG, Heiserman JE. Evidence for cytotoxic edema in the pathogenesis of cerebral venous infarction. AJNR Am J Neuroradiol 2001; 22: 450–455. [PMC free article] [PubMed] [Google Scholar]
- 13. Ducreux D, Oppenheim C, Vandamme X, et al. Diffusion-weighted imaging patterns of brain damage associated with cerebral venous thrombosis. AJNR Am J Neuroradiol 2001; 22: 261–268. [PMC free article] [PubMed] [Google Scholar]
- 14. Appenzeller S, Faria A, Marini R, et al. Focal transient lesions of the corpus callosum in systemic lupus erythematosus. Clin Rheumatol 2006; 25: 568–571. [DOI] [PubMed] [Google Scholar]
- 15. Altunkas A, Aktas F, Ozmen Z, et al. MRI findings of a postpartum patient with reversible splenial lesion syndrome (RESLES). Acta Neurol Belg 2016; 116: 347–349. [DOI] [PubMed] [Google Scholar]
- 16. Takahashi Y, Hashimoto N, Tokoroyama H, et al. Reversible splenial lesion in postpartum cerebral angiopathy: a case report. J Neuroimaging 2014; 24: 292–294. [DOI] [PubMed] [Google Scholar]
- 17. Hackett PH, Yarnell PR, Hill R, et al. High-altitude cerebral edema evaluated with magnetic resonance imaging: clinical correlation and pathophysiology. JAMA 1998; 280: 1920–1925. [DOI] [PubMed] [Google Scholar]