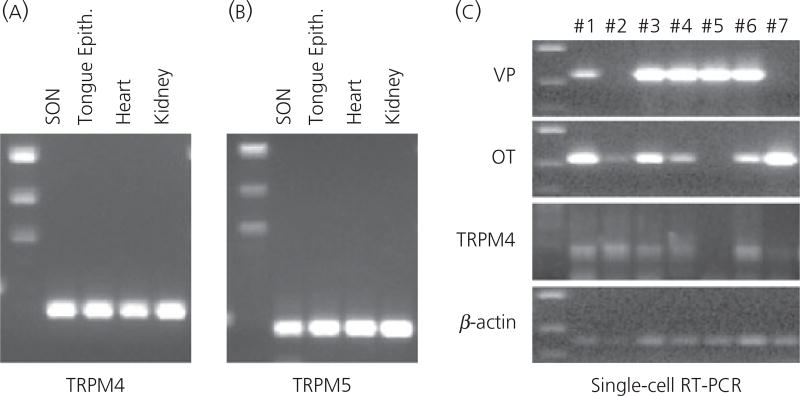
Fig. 7.
(a, b) Reverse transcriptase-polymerase chain reaction (RT-PCR) detection of TRPM4 and TRPM5 in the supraoptic nucleus (SON). The mRNA coding for TRPM4 and TRPM5 is expressed in the SON. Total RNA was extracted from the SON tissue carefully punched out from brain slices, and reverse transcribed. Amplified products of the expected sizes were obtained for TRPM4 (267 bp), TRPM5 (379 bp). Rat kidney, heart and tongue epithelial tissues known to express TRPM4 and/or TRPM5 served as positive controls. Negative control reactions were performed without reverse transcriptase and amplified nothing. (c) Single-cell RT-PCR detection of TRPM4 in the magnocellular cells (MNCs). Individually dissociated MNCs were collected singly from the SON tissue. Following total RNA extraction and RT from these single cells, PCR for vasoporessin (VP) and oxytocin (OT) were performed to verify that the collected cells were MNCs. Either or both OT and VP mRNA were detected in all dissociated cell collected. Because this RT-PCR assay is sensitive, both OT and VP mRNA were detected in the most of MNCs. Because this technique is not quantitative, the cell types of the MNCs should not be determined based on the intensity of the bands on the gel. Nevertheless, this assay demonstrated that these cells are indeed MNCs. The cDNA from each MNC were amplified by PCR for TRPM4 and TRPM5. Amplified products of expected size for TRPM4 were obtained from five out of seven cDNA libraries derived from those single MNCs. The housekeeping gene β-actin was expressed in all MNCs collected, confirming that intact mRNA was isolated from each of these cells.