Fig. 2.
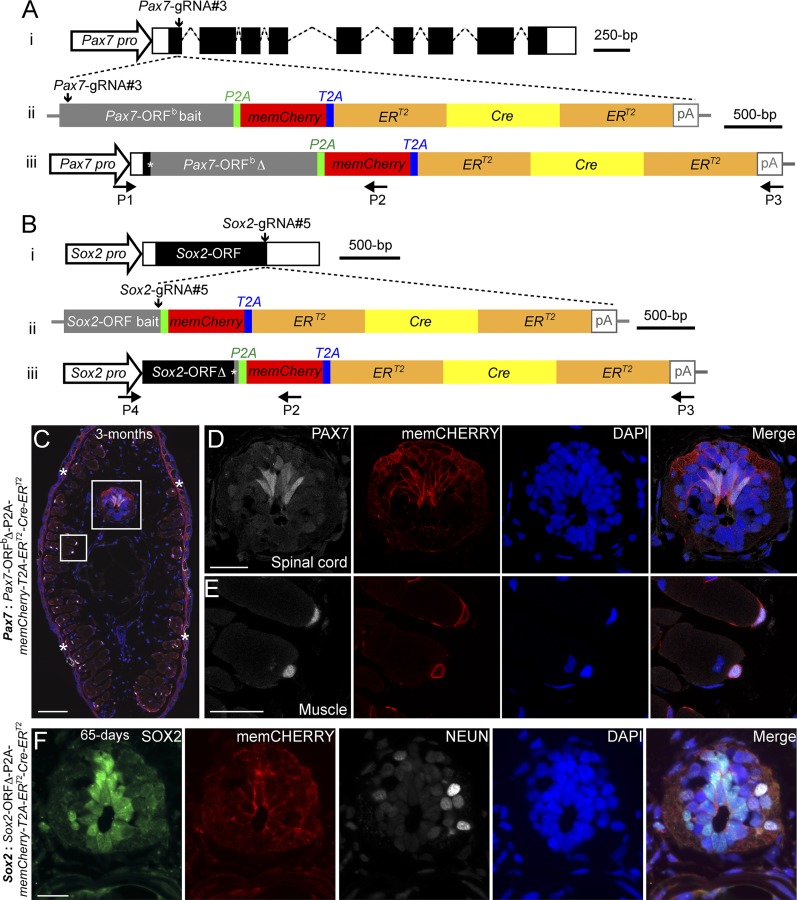
Knockin of a large gene cassette into two axolotl genomic loci through a CRISPR/Cas9- mediated homologous-independent approach. (A and B) The knockin strategies for the generation of the Pax7: Pax7-ORFb∆-P2A-memCherry-T2A-ERT2-Cre-ERT2 (A) and Sox2: Sox2-ORF∆-P2A-memCherry-T2A-ERT2-Cre-ERT2 (B) axolotl transgenic lines. (i) The wild-type axolotl Pax7 (A; Dataset S1) and Sox2 (B) gene structures. Solid rectangles represent coding sequences of the exons; empty rectangles, untranslated regions; dashed lines, introns. Vertical arrows indicate the gRNA targeting sites. (ii) The targeting constructs pGEMT-Pax7-ORFb-P2A-memCherry-T2A- ERT2-Cre-ERT2-PA (A) and pGEMT-Sox2-ORF-P2A-memCherry-T2A-ERT2-Cre-ERT2 -PA (B) contain the entire axolotl Pax7 (A; Pax7-ORFb bait) and Sox2 (B; Sox2-ORF bait) ORF of the cDNA as the bait sequences, followed by the P2A, memCherry (a GAP43 membrane-localization sequence-tagged Cherry gene; Dataset S2), T2A, the ERT2-Cre-ERT2 coding sequences, and the polyadenylation signal (pA). Vertical arrows indicate the gRNA targeting sites. (iii) The Pax7 (A) and Sox2 (B) alleles after successful knockin of the P2A-memCherry-T2A-ERT2-Cre-ERT2 cassettes. Asterisks indicate the junctions after the integration of the targeting constructs. The newly formed mosaic Pax7 (A) and Sox2 (B) coding sequences were designated Pax7-ORFbΔ and Sox2-ORFΔ, respectively. The horizontal arrows indicate the binding positions of the genotyping primers (P1–P4). (C–E) Immunofluorescence for PAX7 (white) and memCHERRY (red) combined with DAPI (blue) on 10-μm tail cross-cryosections of 3-mo-old Pax7: Pax7-ORFbΔ-P2A-memCherry-T2A-ERT2-Cre-ERT2 knockin F0 axolotls shows a membrane-juxtaposed CHERRY signal surrounding PAX7-positive radial glial cells in the spinal cord (C and D) and satellite cells (C and E). The rectangles depicted in C are shown as separated or merged images at higher magnification in D and E. Asterisks in C indicate nonspecific CHERRY staining in the dermis, which is also present in the control (SI Appendix, Fig. S6D). (Scale bars: 200 μm in C, 50 μm in D and E.) (F) Immunofluorescence for SOX2 (green), CHERRY (red, memCHERRY), NEUN (white, to label neurons), and DAPI (blue) on 10-μm cross-cryosections of 65-d-old Sox2: Sox2-ORFΔ-P2A-memCherry-T2A-ERT2-Cre-ERT2 knockin F0 axolotls shows a membrane-juxtaposed CHERRY signal surrounding SOX2-positive radial glial cells in the spinal cord. (Scale bar: 50 μm.)