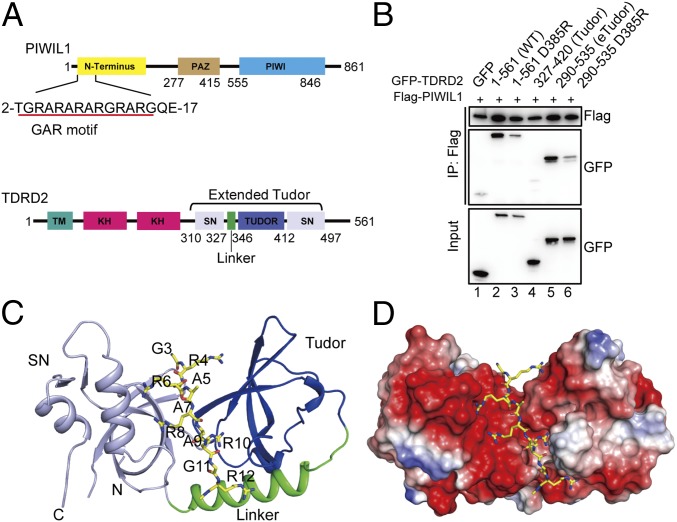
Fig. 1.
The eTudor domain of TDRD2 preferentially recognizes unmethylated PIWIL1. (A, Upper) Domain organization of PIWIL1. (Lower) Domain organization of TDRD2. PAZ, PIWI/Argonaute/Zwille; TM, transmembrane domain. (B) The coimmunoprecipitation assay of PIWIL1 with TDRD2. HEK293T cells were cotransfected with FLAG-PIWIL1 and different GFP-TDRD2 constructs. The complex was analyzed by Western blot with anti-GFP and anti-FLAG antibodies. (C) Overall structure of the eTudor domain of TDRD2 in complex with the PIWIL1 peptide. The color scheme is the same as in A. (D) Electrostatic surface representation of the PIWIL1-binding cleft of TDRD2. PIWIL1 is shown in a stick model.