ABSTRACT
It remains uncertain whether HIV-exposed uninfected (HEU) infants have impaired responses to oral vaccines. We performed a cross-sectional study of 6-month-old infants recruited at birth to the ZVITAMBO trial in Zimbabwe between 1997–2001, before introduction of prevention of mother-to-child transmission interventions. We measured poliovirus-specific IgA to type 1–3 polio strains by semi-quantitative capture ELISA in cryopreserved serum samples collected from 85 HEU and 101 HIV-unexposed infants at 6 months of age, one month after their last immunisation with trivalent OPV. Almost all infants were breastfed, with the majority in both groups mixed breastfed (70.6% HEU versus 71.3% HIV-unexposed). Median (IQR) vaccine titers for HEU and HIV-unexposed infants were 1592 (618–4896) vs. 1774 (711–5431) for Sabin 1 (P = 0.46); 1895 (810–4398) vs. 2308 (1081–4283) for Sabin 2 (P = 0.52); and 1798 (774–4192) vs. 2260 (996–5723) for Sabin 3 (P = 0.18). There were no significant differences in vaccine titers between HEU and HIV-unexposed infants, suggesting that vertical HIV exposure does not impact oral poliovirus vaccine immunogenicity.
KEYWORDS: Africa, HIV, infants, poliovirus, oral vaccine, OPV
Introduction
As coverage of prevention of mother-to-child transmission (PMTCT) increases, the number of HIV-infected infants is declining and there is a growing population of infants in sub-Saharan Africa who are HIV-exposed but uninfected (HEU).1 HEU infants appear to have higher morbidity and mortality and worse growth than HIV-unexposed infants,2 with evidence of altered immunity including diminished antibody responses to specific antigens.3,4 It is therefore plausible that inadequate vaccine responses might contribute to the increased infectious morbidity and mortality reported in HEU infants, although the evidence to date is limited and heterogeneous.5
Oral poliovirus vaccine (OPV) is a live attenuated vaccine, which is inexpensive and easy to administer. By acting in the gastrointestinal tract, OPV can interrupt transmission of the virus and therefore remains a key component of the Polio Eradication and Endgame Strategic Plan.6 However, seroconversion to OPV is lower in developing compared with developed countries,7 which has contributed to the challenges in fully eradicating polio.8 The reasons for this gap in performance are uncertain but may include environmental enteric dysfunction, malnutrition, interference from breast milk antibodies, co-administration with other oral vaccines and concurrent infections.9-13
HIV infection has been associated with significantly lower seroconversion rates to OPV14; however, it is unclear whether HIV exposure contributes to poor oral vaccine immunogenicity. The purpose of this study was therefore to evaluate immune responses to OPV in a well-characterized cohort of HEU and HIV-unexposed infants in Zimbabwe.
Results
A total of 85 HEU and 101 HIV-unexposed infants fulfilled inclusion criteria. Baseline characteristics of infants and their mothers are shown in Table 1. Mothers of HEU infants were older than mothers of HIV-unexposed infants (26.4 vs. 24.6 years, respectively; P = 0.02) and had higher parity (3 vs. 2; P = 0.01); there were no other significant differences between groups. Almost all infants were breastfed, with the majority in both groups mixed breastfed (70.6% HEU vs. 71.3% HIV-unexposed); exclusive breastfeeding was low overall (7.1% vs. 5.0%, respectively).
Table 1.
Baseline characteristics of infants and their mothers.
| HIV exposed uninfected (HEU) N = 85 | HIV-unexposed N = 101 | P value | |
|---|---|---|---|
| Infant Characteristics | |||
| Male sex, % (n) | 56.5 (48) | 52.5 (53) | 0.66 |
| Gestational age, weeks; mean (SD) | 39.4 (1.6) | 39.2 (2.1) | 0.48 |
| Birth weight, kg; mean (SD) | 2.99 (0.47) | 3.01 (0.44) | 0.77 |
| Birth length, cm; mean (SD) | 48.4 (2.7) | 47.8 (2.4) | 0.11 |
| Birth head circumference, cm; mean (SD) | 34.2 (2.2) | 34.1 (2.1) | 0.75 |
| Normal vaginal delivery, % (n) | 87.0 (74) | 86.7 (85) [98] | 0.68 |
| Exclusive breast feeding1, % (n) | 7.1 (6) | 5.0 (5) | 0.55 |
| Predominant breast feeding, % (n) | 22.4 (19) | 23.8 (24) | 0.86 |
| Mixed feeding, % (n) | 70.6 (60) | 71.3 (72) | 1.00 |
| Maternal characteristics | |||
| Age, years; mean (SD) | 26.4 (4.9) | 24.6 (5.7) | 0.02 |
| Married or stable union, % (n) | 91.8 (78) | 95.0 (96) | 0.39 |
| Education, years; median (IQR) | 10 (7,11) | 11 (9,11) | 0.14 |
| Parity, median (IQR) | 3 (2,3) | 2 (1,3) | 0.01 |
| Maternal MUAC, cm; mean (SD) | 26.4 (2.8) | 26.4 (3.1) | 1.00 |
| Employed, % (n) | 77.6 (66) | 86.1 (87) | 0.18 |
| Monthly household income, USD; mean (SD) | 7.83 (6.24) | 7.31 (5.56) | 0.56 |
[x] refers to total number if data missing
Breastfeeding status assessed at 6-months postpartum
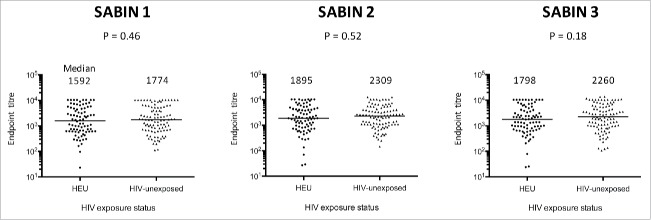
At 6 months of age (1 month post-immunisation), median (IQR) vaccine titers for HEU and HIV-unexposed infants were 1592 (618–4896) vs. 1774 (711–5431) for Sabin 1 (P = 0.46); 1895 (810–4398) vs. 2308 (1081–4283) for Sabin 2 (P = 0.52); and 1798 (774–4192) vs. 2260 (996–5723) for Sabin 3 (P = 0.18) (Fig. 1). Differences in log mean titers between HEU and HIV-unexposed groups were similar after adjusting for breastfeeding, birth weight and sex in a linear regression model (P = 0.36, Sabin 1; P = 0.33, Sabin 2; P = 0.19, Sabin 3).
Figure 1.
Serum polio-specific IgA end point titers (Sabin 1,2 and 3) at 6 months of age in HIV-exposed uninfected (HEU) and HIV-unexposed infants.
Discussion
Although immunological abnormalities have been described in HIV-exposed uninfected infants,5 our results suggest that immune responses to OPV were similar among HEU and HIV-unexposed Zimbabwean infants at 6 months of age, adding to the growing body of literature on vaccine responses in HEU infants.1
Most prior studies have evaluated responses to parenteral vaccines, generally finding similar (or even better) responses among HEU compared with HIV-unexposed infants.15-18 Few studies to date have evaluated responses to oral vaccines, and only 3 to our knowledge have compared OPV responses.19-21 A study of HEU infants in Zambia showed lower mean OPV 2 neutralising antibody titers at 18 months of age compared with HIV-unexposed infants, although this was not significant after adjusting for differences in breastfeeding.20 HEU infants in Cameroon and Central African Republic aged 18–35 months showed high rates of seroconversion to trivalent OPV (96–100%).21 A study from Malawi found no difference in median anti-polio immunoglobulin G (IgG) titers between HEU and HIV-unexposed infants at 10 weeks of age, but only included 34 infants.19 Our study of HEU infants from Zimbabwe is consistent with these prior studies, showing that OPV was equally immunogenic in HEU and HIV-unexposed infants.
A major strength of this study is the well-characterized cohort of HEU infants born in the pre-PMTCT era together with a demographically similar HIV-unexposed population. However, there are a few important limitations. Firstly, immunisation data were not collected as part of the original ZVITAMBO trial, so we were unable to confirm the number of OPV doses received by infants. However, we have no reason to believe that HEU infants would be less likely to complete the EPI schedule than HIV-unexposed infants, and in fact clinic attendance for intercurrent illnesses was higher among HEU infants.22 Secondly, our sample size was limited by the number of cryopreserved specimens with sufficient volume still remaining from the original trial. We may have therefore been underpowered to pick up differences between groups. Thirdly, while the ELISA we used to detect polio-specific IgA enables a comparison of immunogenicity between groups, we did not have access to the gold-standard OPV neutralization assay, which provides a measure of vaccine protection. Fourth, without available samples to measure baseline IgA titers, we cannot rule out a difference between groups in antibody rise between pre- and post-vaccination. Finally, we only measured antibody responses shortly after immunisation. There is some evidence that the quality and quantity of vaccine-specific antibody declines faster in HEU compared with HIV-unexposed infants23 so we cannot comment on durability of protection.
In summary, in a well-characterized cohort of HEU and HIV-unexposed infants, recruited before PMTCT introduction, we found that HIV exposure does not appear to affect serum IgA responses to OPV. In an era when new oral vaccines, such as rotavirus, are being introduced in areas of high antenatal HIV prevalence, this is reassuring, although further studies are required to ascertain the reasons for the recognized underperformance of oral vaccines in developing countries.
Materials and methods
Study design and population
This study utilised archived samples from the Zimbabwe Vitamin A for Mothers and Babies (ZVITAMBO) trial, which has been described previously.24 Briefly, 14110 mother-infant pairs were enrolled within 96 hours of delivery in Harare, Zimbabwe between 1997 and 2001. Mother-infant pairs were eligible if neither had an acutely life-threatening condition and the infant was a singleton with birth weight >1500 g. Written informed consent was obtained. Socioeconomic and demographic information was collected by maternal interview. Follow-up was conducted at 6 weeks, 3 months and then 3 monthly to 12–24 months of age. The trial was conducted in a peri-urban setting and preceded availability of antiretroviral therapy in Zimbabwe or use of cotrimoxazole prophylaxis for HIV-exposed infants. Anthropometry was conducted at each visit, using methods and WHO reference standards as described previously.25
Biological specimen collection
Blood was collected by venipuncture from all mothers and infants at baseline (≤96 hours after delivery) and at all follow-up visits. Samples were centrifuged and plasma removed within 2 hours of collection. Samples were stored in −80°C freezers with automatic generator backup
Ascertainment of HIV exposure status
Mothers underwent HIV testing at baseline using 2 parallel ELISA assays. Women testing HIV-negative were re-tested at every visit to detect HIV seroconversion. The last available sample from each child was tested for HIV by GeneScreen ELISA on plasma if aged ≥ 18 months, or by DNA polymerase chain reaction (Roche Amplicor version 1.5; Roche Diagnostic Systems, Alameda, CA) in cell pellets if aged <18 months. If the last available sample was negative, the child was classified as HIV-negative. Children were classified as HIV-unexposed if the mother tested HIV-negative at baseline and did not seroconvert during follow-up; children were classified as HIV-exposed uninfected (HEU) if the mother tested HIV-positive at baseline and the last available infant sample was HIV-negative. Infants of mothers who seroconverted during follow up were not included.
Selection of infants
For the current cross-sectional study, we retrieved samples for all HEU and HIV-unexposed infants fulfilling the following criteria: 1) Mother and infant both received placebo in the original trial; 2) infants were followed to 6 months with available feeding and anthropometry data; and 3) a sufficient sample of cryopreserved serum was available at 6 months of age.
Infant feeding counselling
All mothers, irrespective of HIV status, were encouraged to exclusively breastfeed their infants for 6 months. Data on feeding practices at 6 weeks and 3 months were used to categorise infants as exclusively, predominantly or mixed breastfed, as previously defined.26
Oral poliovirus vaccination
Infants followed the routine Expanded Programme of Immunisation schedule in Zimbabwe at the time, which included trivalent OPV at 3, 4 and 5 months of age. Specific vaccination data were not collected as part of the trial, but OPV3 immunisation coverage (collated by WHO using data from routine reporting systems) at that time in Zimbabwe was 70–81%.27
Antigen-capture ELISA for detection of poliovirus IgA
Although neutralising antibody titer is the best correlate of protection for OPV,28,29 measurements of mucosal (salivary/stool secretory IgA) and circulating (serum) IgA responses to poliovirus are useful in the detection and control of poliovirus infection,30-35 particularly in settings without access to a WHO-accredited polio reference laboratory capable of performing neutralization assays.
In all 6-month serum samples (i.e. one month after receipt of final OPV), we measured poliovirus-specific IgA to type 1–3 polio strains in a capture ELISA developed by collaborators at the Centers for Disease Control and Prevention (CDC) as described previously.36 First, 96-well microplates were coated with goat anti-human IgA (SeraCare, Massachusetts) for 60 minutes at 37°C. Next plates were washed with buffer containing phosphate buffered saline (PBS) and 0.05% Tween 20 and incubated for a further 60 minutes with dilution buffer containing bovine serum albumin dissolved in PBS (all purchased from Sigma, St Louis). We then made 3-fold serial dilutions of serum samples beginning at a dilution of 1 in 100 and added 50 uL of diluted sample to each well (one patient sample per column). After a further wash step, the plates were incubated overnight in a moist chamber at room temperature with Sabin antigen, cultured from Hep2C cells at the University of Vermont. The next day, the plates were washed followed by addition of monoclonal Sabin antibody (Merck Millipore, Massachusetts) to the corresponding Sabin antigen and incubated at 37°C for 60 minutes. After another wash step, goat anti-mouse IgG conjugated to enzyme (SeraCare) was added to the plates for a further 60 minutes at 37°C. We then developed the reactions by addition of substrate for 15 minutes at room temperature, with the output being optical densitiy (O.D.) at 450 nm. Pooled serum from poliovirus-vaccinated healthy donor volunteers was used as a positive control; each plate also included a blank column with no test sample as a negative control.
Using 3-fold serial dilutions of patient samples run out in a single column allowed for a semi-quantitative measure of poliovirus-specific IgA, an adaptation of the original method. Endpoint titers were calculated by subtracting the plate background (i.e., the average O.D. of the negative control wells), then identifying the dilution of the final well in each sample column with an O.D. >0.07 (a 95% confidence value cut-off used to distinguish “negative” from “positive” absorbance values and calculated according to the original method36). The intra- and inter-plate coefficients of variation for Sabin 1, 2 and 3 strains, derived from the mean O.D.s and standard deviations from 7 successive preliminary experiments run before including study samples, were 8.2%, 4.0% and 4.8% and 20.1%, 17.7% and 16.8%, respectively. As a means of continued quality assurance, the end point titer of the positive control sample in all subsequent assays had to fall within an acceptable range for the experiment to be deemed valid. The range was pre-determined based on the upper and lower limit O.D.s in the 7 preliminary experiments. Laboratory scientists were blinded to infant HIV exposure status when conducting the assays.
If an end point titer could not be derived at the first attempt (e.g. bottom well O.D. ≥0.08), the assay was repeated using a 10-fold higher or lower concentration of patient sample where sufficient serum volume was available. If an end point titer could not be derived using the new sample concentration, the lowest or highest dilution factor was taken as the final end point. Extreme low and high end point titers were subsequently truncated and assigned a value equivalent to the 5th and 95th centile within the data set respectively.
Statistical analysis
Baseline characteristics were compared between groups using Fisher's exact tests for categorical variables, and Mann-Whitney or 2-sample t-tests for continuous variables, depending on data distribution. A regression model was used to calculate adjusted differences between median vaccine titers between groups, using breastfeeding, birth weight and infant sex as covariates. Statistical analyses were performed using STATA 13 (Stata-Corp, College Station, TX, USA) and Prism version 6 (GraphPad Software Inc., La Jolla, CA, USA).
Ethics statement
The original ZVITAMBO trial and this laboratory substudy were approved by the Medical Research Council of Zimbabwe, John Hopkins School of Public Health Committee on Human Research and the Montreal General Hospital Ethics Committee.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank the mothers and babies who participated in the ZVITAMBO trial, and the ZVITAMBO Study Group. JAC, MPC, SAD conceived and designed the experiments. JAC, MG, SR performed the experiments. JAC, BC, RN and AJP analyzed the data. JAC, MPC, SAD, RN, JH, BDK and AJP wrote the paper. We are also grateful to WC Weldon for his helpful comments on this manuscript and his work with AJ Williams at the Centers for Disease Control and Prevention in developing the Polio IgA capture ELISA method.
Funding
The ZVITAMBO trial was supported by the Canadian International Development Agency (CIDA) (R/C Project 690/M3688), United States Agency for International Development (USAID) (cooperative agreement number HRN-A-00–97–00015–00 between Johns Hopkins University and the Office of Health and Nutrition – USAID) and a grant from the Bill and Melinda Gates Foundation, Seattle WA. Additional funding was received from the SARA Project, which is operated by the Academy for Educational Development, Washington DC and is funded by USAID's Bureau for Africa, Office of Sustainable Development under the terms of Contract AOT-C-00–99–00237–00, the Rockefeller Foundation (NY, NY) and BASF (Ludwigshafen, Germany). This current study was supported by the Sanitation & Hygiene Applied Research for Equity (SHARE) Consortium and Barts Charity (grant number 212563 to JAC). JAC (Grant 201293/Z/16/Z) and AJP (Grant 108065/Z/15/Z) are supported by the Wellcome Trust.
References
- [1].Evans C, Jones CE, Prendergast AJ. HIV-exposed, uninfected infants: new global challenges in the era of paediatric HIV elimination. Lancet Infect Dis. 2016;16(6):e92-e107. doi: 10.1016/S1473-3099(16)00055-4. PMID:27049574 [DOI] [PubMed] [Google Scholar]
- [2].Filteau S. The HIV-exposed, uninfected African child. Trop Med Int Health. 2009;14(3):276-87. doi: 10.1111/j.1365-3156.2009.02220.x. PMID:19171011 [DOI] [PubMed] [Google Scholar]
- [3].Bernstein LJ, Ochs HD, Wedgwood RJ, Rubinstein A. Defective humoral immunity in pediatric acquired immune deficiency syndrome. J Pediatr. 1985;107(3):352-7. doi: 10.1016/S0022-3476(85)80505-9. PMID:4032129 [DOI] [PubMed] [Google Scholar]
- [4].Shearer WT, Easley KA, Goldfarb J, Rosenblatt HM, Jenson HB, Kovacs A, McIntosh K. Prospective 5-year study of peripheral blood CD4, CD8, and CD19/CD20 lymphocytes and serum Igs in children born to HIV-1 women. The P(2)C(2) HIV Study Group. J Allergy Clin Immunol. 2000;106(3):559-66. doi: 10.1067/mai.2000.109433. PMID:10984378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Afran L, Garcia Knight M, Nduati E, Urban BC, Heyderman RS, Rowland-Jones SL. HIV-exposed uninfected children: a growing population with a vulnerable immune system? Clin Exp Immunol. 2014;176(1):11-22. doi: 10.1111/cei.12251. PMID:24325737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Polio Eradication & Endgame Strategic Plan 2013-2018; Polio Global Eradication Initiative. Geneva: World Health Organisation; 2013 [accessed 2016 Nov 8]. Available at: http://polioeradication.org/wp-content/uploads/2016/07/PEESP_EN_A4.pdf [Google Scholar]
- [7].Patriarca PA, Wright PF, John TJ. Factors affecting the immunogenicity of oral poliovirus vaccine in developing countries: review. Rev Infect Dis. 1991;13(5):926-39. doi: 10.1093/clinids/13.5.926. PMID:1660184 [DOI] [PubMed] [Google Scholar]
- [8].Grassly NC, Jafari H, Bahl S, Durrani S, Wenger J, Sutter RW, Bruce Aylward R. Asymptomatic wild-type poliovirus infection in India among children with previous oral poliovirus vaccination. J Infect Dis. 2010;201(10):1535-43. doi: 10.1086/651952. PMID:20367459 [DOI] [PubMed] [Google Scholar]
- [9].Haque R, Snider C, Liu Y, Ma JZ, Liu L, Nayak U, Mychaleckyj JC, Korpe P, Mondal D, Kabir M, et al.. Oral polio vaccine response in breast fed infants with malnutrition and diarrhea. Vaccine. 2014;32(4):478-82. doi: 10.1016/j.vaccine.2013.11.056. PMID:24300591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Maldonado YA, Peña-Cruz V, de la Luz Sanchez M, Logan L, Blandón S, Cantwell MF, Matsui SM, Millan-Velasco F, Valdespino JL, Sepulveda J. Host and viral factors affecting the decreased immunogenicity of Sabin type 3 vaccine after administration of trivalent oral polio vaccine to rural Mayan children. J Infect Dis. 1997;175(3):545-53. doi: 10.1093/infdis/175.3.545. PMID:9041324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Posey DL, Linkins RW, Oliveria MJ, Monteiro D, Patriarca PA. The effect of diarrhea on oral poliovirus vaccine failure in Brazil. J Infect Dis. 1997;175 Suppl 1:S258-63. doi: 10.1093/infdis/175.Supplement_1.S258. PMID:9203726 [DOI] [PubMed] [Google Scholar]
- [12].Triki H, MV Abdallah, Ben Aissa R, Bouratbine A, Ben Ali Kacem M, Bouraoui S, Koubaa C, Zouari S, Mohsni E, Crainic R, et al.. Influence of host related factors on the antibody response to trivalent oral polio vaccine in Tunisian infants. Vaccine. 1997;15(10):1123-9. doi: 10.1016/S0264-410X(97)00001-7. PMID:9269056 [DOI] [PubMed] [Google Scholar]
- [13].Naylor C, Lu M, Haque R, Mondal D, Buonomo E, Nayak U, Mychaleckyj JC, Kirkpatrick B, Colgate R, Carmolli M, et al.. Environmental enteropathy, oral vaccine failure and growth faltering in infants in Bangladesh. EBioMedicine. 2015;2(11):1759-66. doi: 10.1016/j.ebiom.2015.09.036. PMID:26870801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Troy SB, Musingwini G, Halpern MS, Huang C, Stranix-Chibanda L, Kouiavskaia D, Shetty AK, Chumakov K, Nathoo K, Maldonado YA. Vaccine poliovirus shedding and immune response to oral polio vaccine in HIV-infected and -uninfected Zimbabwean infants. J Infect Dis. 2013;208(4):672-8. doi: 10.1093/infdis/jit208. PMID:23661792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Jones CE, Naidoo S, De Beer C, Esser M, Kampmann B, Hesseling AC. Maternal HIV infection and antibody responses against vaccine-preventable diseases in uninfected infants. JAMA. 2011;305(6):576-84. doi: 10.1001/jama.2011.100. PMID:21304083 [DOI] [PubMed] [Google Scholar]
- [16].Madhi SA, Izu A, Violari A, Cotton MF, Panchia R, Dobbels E, Sewraj P, van Niekerk N, Jean-Philippe P, Adrian PV. Immunogenicity following the first and second doses of 7-valent pneumococcal conjugate vaccine in HIV-infected and -uninfected infants. Vaccine. 2013;31(5):777-83. doi: 10.1016/j.vaccine.2012.11.076. PMID:23228814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Reikie BA, Naidoo S, Ruck CE, Slogrove AL, de Beer C, la Grange H, Adams RC, Ho K, Smolen K, Speert DP, et al., Antibody responses to vaccination among South African HIV-exposed and unexposed uninfected infants during the First 2 years of life. Clin Vaccine Immunol. 2013;20(1):33-8. doi: 10.1128/CVI.00557-12. PMID:23114697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Simani OE, Izu A, Violari A, Cotton MF, van Niekerk N, Adrian PV, Madhi SA. Effect of HIV-1 exposure and antiretroviral treatment strategies in HIV-infected children on immunogenicity of vaccines during infancy. Aids. 2014;28(4):531-41. doi: 10.1097/QAD.0000000000000127. PMID:24468996 [DOI] [PubMed] [Google Scholar]
- [19].Miles DJ, Gadama L, Gumbi A, Nyalo F, Makanani B, Heyderman RS. Human immunodeficiency virus (HIV) infection during pregnancy induces CD4 T-cell differentiation and modulates responses to Bacille Calmette-Guerin (BCG) vaccine in HIV-uninfected infants. Immunology. 2010;129(3):446-54. doi: 10.1111/j.1365-2567.2009.03186.x. PMID:20002789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sanz-Ramos M, Manno D, Kapambwe M, Ndumba I, Musonda KG, Bates M, Chibumbya J, Siame J, Monze M, Filteau S, et al.. Reduced Poliovirus vaccine neutralising-antibody titres in infants with maternal HIV-exposure. Vaccine. 2013;31(16):2042-9. doi: 10.1016/j.vaccine.2013.02.044. PMID:23474309 [DOI] [PubMed] [Google Scholar]
- [21].Tejiokem MC, Gouandjika I, Béniguel L, Zanga MC, Tene G, Gody JC, Njamkepo E, Kfutwah A, Penda I, Bilong C, et al.. HIV-Infected children living in Central Africa have low persistence of antibodies to vaccines used in the expanded program on immunization. Plos One. 2007;2(12):e1260. doi: 10.1371/journal.pone.0001260. PMID:18060056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Koyanagi A, Humphrey JH, Ntozini R, Nathoo K, Moulton LH, Iliff P, Mutasa K, Ruff A, Ward B. Morbidity among human immunodeficiency virus-exposed but uninfected, human immunodeficiency virus-infected, and human immunodeficiency virus-unexposed infants in Zimbabwe before availability of highly active antiretroviral therapy. Pediatr Infect Dis J. 2011;30(1):45-51. doi: 10.1097/INF.0b013e3181ecbf7e. PMID:21173675 [DOI] [PubMed] [Google Scholar]
- [23].Gaensbauer JT, Rakhola JT, Onyango-Makumbi C, Mubiru M, Westcott JE, Krebs NF, Asturias EJ, Fowler MG, McFarland E, Janoff EN. Impaired Haemophilus influenzae Type b Transplacental antibody transmission and declining antibody avidity through the first year of life represent Potential Vulnerabilities for HIV-Exposed but - uninfected infants. Clin Vaccine Immunol. 2014;21(12):1661-7. doi: 10.1128/CVI.00356-14. PMID:25298109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Marinda E, Humphrey JH, Iliff PJ, Mutasa K, Nathoo KJ, Piwoz EG, Moulton LH, Salama P, Ward BJ. Child mortality according to maternal and infant HIV status in Zimbabwe. Pediatr Infect Dis J. 2007;26(6):519-26. doi: 10.1097/01.inf.0000264527.69954.4c. PMID:17529870 [DOI] [PubMed] [Google Scholar]
- [25].Gibson RS. Principles of Nutritional Assessment. Second ed Oxford, UK: Oxford University Press; 1990. p. 712. [Google Scholar]
- [26].Iliff PJ, Piwoz EG, Tavengwa NV, Zunguza CD, Marinda ET, Nathoo KJ, Moulton LH, Ward BJ, Humphrey JH. Early exclusive breastfeeding reduces the risk of postnatal HIV-1 transmission and increases HIV-free survival. AIDS. 2005;19(7):699-708. doi: 10.1097/01.aids.0000166093.16446.c9. PMID:15821396 [DOI] [PubMed] [Google Scholar]
- [27].WHO Immunization coverage: reported coverage by country, year and vaccine. 15thJuly2016 24thOctober2016]; Immunization coverage per country, year and disease dating back to 1980]. Available from: http://apps.who.int/immunization_monitoring/globalsummary/timeseries/tscoveragebcg.html.
- [28].Ivanov AP, Dragunsky EM. ELISA as a possible alternative to the neutralization test for evaluating the immune response to poliovirus vaccines. Expert Rev Vaccines. 2005;4(2):167-72. doi: 10.1586/14760584.4.2.167. PMID:15889990 [DOI] [PubMed] [Google Scholar]
- [29].Simhon A, Lifshitz A, Abed Y, Lasch EE, Schoub B, Morag A. How to Predict the Immune Status of Poliovirus Vaccinees - a Comparison of Virus Neutralization at a Very Low Serum Dilution Versus Elisa in a Cohort of Infants. Int J Epidemiol. 1990;19(1):164-8. doi: 10.1093/ije/19.1.164. PMID:2161806 [DOI] [PubMed] [Google Scholar]
- [30].Gary BE, Freeman C, Peñaranda S, Maher K, Anderson L, Pallansch MA. Comparison of a monoclonal antibody-based IgM capture ELISA with a neutralization assay for assessing response to trivalent oral poliovirus vaccine. J Infect Dis. 1997;175:S264-7. doi: 10.1093/infdis/175.Supplement_1.S264. PMID:9203727 [DOI] [PubMed] [Google Scholar]
- [31].Hashido M, Horie H, Abe S, Doi Y, Hashizume S, Agboatwalla M, Isomura S, Nishio O, Hagiwara A, Inouye S. Evaluation of an enzyme-linked immunosorbent assay based on binding inhibition for type-specific quantification of poliovirus neutralization-relevant antibodies. Microbiol Immunol. 1999;43(1):73-7. doi: 10.1111/j.1348-0421.1999.tb02375.x. PMID:10100750 [DOI] [PubMed] [Google Scholar]
- [32].Herremans T, Kimman TG, Conyn-Van Spaendonck MA, Buisman A, de Melker H, Abbink F, Bijkerk P, Koopmans MP. Immunoglobulin A as a serological marker for the (silent) circulation of poliovirus in an inactivated poliovirus-vaccinated population. Clin Infect Dis. 2002;34(8):1067-75. doi: 10.1086/339489. PMID:11914995 [DOI] [PubMed] [Google Scholar]
- [33].Wright PF, Wieland-Alter W, Ilyushina NA, Hoen AG, Arita M, Boesch AW, Ackerman ME, van der Avoort H, Oberste MS, Pallansch MA, et al.. Intestinal immunity is a determinant of clearance of poliovirus after oral vaccination. J Infect Dis. 2014;209(10):1628-34. doi: 10.1093/infdis/jit671. PMID:24459191 [DOI] [PubMed] [Google Scholar]
- [34].Smith DJ, Gahnberg L, Taubman MA, Ebersole JL. Salivary antibody responses to oral and parenteral vaccines in children. J Clin Immunol. 1986;6(1):43-9. doi: 10.1007/BF00915363. PMID:3958136 [DOI] [PubMed] [Google Scholar]
- [35].Hird TR, Grassly NC. Systematic review of mucosal immunity induced by oral and inactivated poliovirus vaccines against virus shedding following oral poliovirus challenge. PLoS Pathog. 2012;8(4):e1002599. doi: 10.1371/journal.ppat.1002599. PMID:22532797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Weldon WC, Oberste MS, Pallansch MA. Standardized methods for detection of poliovirus antibodies. Methods Mol Biol. 2016;1387:145-76. doi: 10.1007/978-1-4939-3292-4_8 [DOI] [PubMed] [Google Scholar]